Oil refineries produce fuel. But the cost in liters at gas stations is so daunting that car owners often ask how they can make gasoline at home. Every year, heaps of waste are released into the atmosphere. And if you put them to work, select a unit for distillation, then you can completely try to make methanol (methyl alcohol) on your own.
What is methanol and how to make it
Methanol is a poisonous colorless solvent with the taste of drinking alcohol, the octane number of which is 150. Essentially, it is the same fuel. Although it differs from motor gasoline in that after filling:
- Increases engine power by 20%, service life several times
- Does not emit harmful components when the engine is running. This means it is an environmentally friendly product.
How to make gasoline from methanol in artisanal conditions? It is possible using moonshine or denaturation technology (adding naphtha, kerosene). The process of methanol distillation is a step-by-step process, which differs from the distillation of moonshine. The output should be clean fuel with the least amount of water. During production, it will be necessary to install cleaning filters to the unit that will remove excess liquid from fuel alcohol.
Ethanol is a solvent. It will wash corroded dirt from the fuel lines into the cylinders. This means that the filters will serve as a separator of debris and water from the fuel tank.
Step-by-step steps for making homemade gasoline from methanol:
- Selection of initial raw materials for making mash (wheat, corn, millet, Jerusalem artichoke).
- Combining foods with sugar to begin the fermentation process, then fermentation to produce alcohol.
- Selection of a unit made of stainless steel or iron. To obtain 3 liters of gasoline in 1 hour, it is enough to select thin copper tubes of the following dimensions: width - 30 cm, length - 50 cm, height - 20 cm, diameter - 75 mm. A capillary tube from a used refrigerator is suitable as a faucet, and a pressure reducing valve is from a gas cylinder.
- Installation of a mixer with a reactor horizontally for heating.
- Connecting the structure to a water supply system divided into 2 streams. One will go into the refrigerator through a faucet and hole. Another is to enter the faucet through a faucet with a hole.
Water will flow through the hole, begin to cool, and turn into condensate and synthesis gas. Natural gas connected to the pipeline goes into the mixer, mixes with water steam, heats up to t +120 degrees. using a torch
It is important to adjust the faucet immediately. This will maintain optimal pressure in the condenser. You can't close it completely. It is enough to open it slightly for water to start flowing. When the temperature in the reactor reaches +250, you should open the tap a little more so that gasoline flows out in a thin stream. Still open slightly in case of leakage of fuel with gas impurities.
The unit is connected to a gas burner and adjusted to high performance. It is important that the least amount of steam is formed in the mixer, and that there is practically no water left in the fuel at the outlet. You can check the content with an alcohol meter. A pressure gauge is attached to the hole on the condenser to keep the pressure under control within 10 atm.
Tap water contains chlorine. This means that it will instantly lead to poisoning of the catalyst of the second reactor. That is why many craftsmen pour distilled water into the installation (reactor). The gas also contains sulfur impurities and active organic compounds. To achieve better results, it is better to use monoethanolamine gas purification.
Solid and gaseous fuels edit code
In some third world countries, firewood and charcoal are still the main fuel available to the population for heating and cooking (about half of the world's population lives this way). This in many cases leads to deforestation, which in turn leads to desertification and soil erosion. One of the ways to reduce the population's dependence on wood sources is to introduce technology for briquetting agricultural waste or household waste into fuel briquettes. Such briquettes are produced by pressing a slurry obtained by mixing waste with water on a simple lever press, followed by drying. This technology, however, is very labor-intensive and requires the availability of a source of cheap labor. A less primitive option for producing briquettes is to use hydraulic pressing machines.
Some gaseous fuels can be considered variants of synthetic fuels, although this definition may be controversial since engines using such fuels require significant modification. One of the widely discussed options for reducing the contribution of motor vehicles to the accumulation of carbon dioxide in the atmosphere is the use of hydrogen as a fuel. Hydrogen engines do not pollute the environment and emit only water vapor. Hydrogen-oxygen fuel cells use hydrogen to directly convert the energy of a chemical reaction into electrical energy. Since hydrogen is produced either by methods that require large amounts of electricity, or by the oxidation of hydrocarbon fuels, the environmental and, especially, economic advantages of such fuel are highly controversial.
Full article Hydrogen energy
.
Dimethyl etheredit | edit code
Dimethyl ether is obtained by dehydration of methanol at 300-400 °C and 2-3 MPa in the presence of heterogeneous catalysts - aluminosilicates. The degree of conversion of methanol into dimethyl ether is 60%, into zeolites - almost 100%. Dimethyl ether is an environmentally friendly fuel without sulfur content, and the emission of nitrogen oxides in exhaust gases is 90% less than that of gasoline. The cetane number of dimethyl diesel is more than 55, while that of classic petroleum diesel is from 38 to 53. The use of dimethyl ether does not require special filters, but it requires alteration of the power supply systems (installation of gas equipment, adjustment of mixture formation) and engine ignition. Without modification, it can be used on cars with LPG engines with a 30% methanol content in the fuel.
The calorific value of DME is about 30 MJ/kg, for classical petroleum fuels it is about 42 MJ/kg. One of the features of the use of DME is its higher oxidizing ability (due to the oxygen content) than that of classical fuel.
In July 2006, the National Development and Reform Commission (NDRC) (China) adopted a standard for the use of dimethyl ether as a fuel. The Chinese government will support the development of dimethyl ether as a possible alternative to diesel fuel. In the next 5 years, China plans to produce 5-10 million tons of dimethyl ether per year.
Cars with engines running on dimethyl ether are being developed by KAMAZ, Volvo, Nissan and the Chinese company Shanghai Automotive.
Homemade gasoline options
Producing real fuel is a complex, costly process. First, oil is extracted and then redirected for processing. It is hardly possible to produce high-quality gasoline at home. However, there is enough fuel for heating or refueling a chainsaw.
Petroleum products are found everywhere. This is garbage under people's feet (synthetic fabrics, polyethylene, plastic, rubber tires, rotten wood). From them, diesel fuel can be extracted for internal combustion engines if it is subjected to pyrolysis or heating under oxygen-free conditions. Recyclable materials will release carbon dioxide when burned. This means it will not harm the external environment. The output will be fuel no worse than gasoline from a gas station.
1 kg of bottle plastic yields 1 liter of fuel. You can make homemade gasoline in artisanal conditions from coal by heat treatment, old tires, rubber waste, crude oil and gasification. Some craftsmen have learned to extract gasoline for internal combustion engines. Good synthetic diesel fuel is produced by distilling plastic bottles and rubber tires.
Not just gasoline or anything else you can fill your car with.
At the dawn of the automotive industry, low-power, but simple and unpretentious internal combustion engines consumed almost any liquid fuel. The absence in those days of modern developed networks of gas stations forced motorists to pour kerosene, solvent, and even flammable rodent repellent into the tank of their car.
Modern car enthusiasts, especially residents of European countries, are much less likely to encounter such difficulties. True, car owners living in the territory of post-Soviet states often carry a small canister or an ordinary two-liter plastic bottle of gasoline in the trunk of their car, and such foresight has more than once helped them out in difficult situations.
However, what to do if the supply of gasoline is used up, and you can’t get to the nearest gas station with a canister in your hands? Can a modern car travel a certain distance using flammable liquids as fuel, which can be easily obtained in any village, and how much will this affect the performance of a modern engine?
To carry out the experiment, the fuel system of the Niva equipped with injection was somewhat modernized, so that fuel began to come from a small portioned tank located directly under the hood. Paired with the new “gas tank,” a separate fuel pump was installed. On the one hand, this ensured the purity of the experiment, excluding gasoline residues from getting into the test flammable liquids. On the other hand, it left the possibility of starting the car on regular gasoline if none of the tested liquids worked.
Kerosene, turpentine, two brands of solvent, acetone, propane-butane, diesel fuel and various alcoholic drinks with a high alcohol content were chosen as possible substitutes for gasoline. We also paid attention to the so-called “anti-freeze”, which burns well due to the high content of various alcohols. Before testing each new type of fuel, the remaining old fuel in the system was thoroughly washed out.
The car had to travel 1 kilometer on the tested non-standard fuel.
The Autoass motor tester was used as measuring equipment. The level of fuel optimality was determined by injection duration, and power was assessed by measuring the time the engine reached 3000 rpm.
However, personal impressions of the behavior of a car engine turned out to be much more interesting and complete than the accurate but dry instrument readings, and from the outside, a special impression was made by the Niva in a test run on liquefied gas - a rubber hose going from the passenger compartment to the engine compartment, dangling under the pressure of the headwind - a spectacle not for the faint of heart.
Making gasoline from coal
European countries, not having oil reserves, have long started producing gasoline from raw coal. Also in the pre-war years, Germany produced fuel thanks to the Ruhr coal basin with its large deposits of brown coal
How gasoline is separated from coal
Oil and coal have identical chemical compositions (hydrogen, carbon compounds), which can simply be replaced. This means that as the number of hydrogen molecules in coal increases or hydrogenates, oil will begin to be released first, then gasoline will be released through processing.
The separation of fuel from oil is possible in two ways: Fischer-Tropsch, Bergius by synthesis and gasification of fuel or liquefaction (hydrogenation).
Definition of the term synthetic fuel code
The term "synthetic fuel" has several different meanings and can include different types of fuel. The traditional definition set by the International Energy Agency defines "synthetic fuels" as any liquid fuel derived from coal or natural gas. The US Energy Information Association defines synthetic fuels in its 2006 annual report as fuels derived from coal, natural gas, biomass or animal feed by chemical conversion into synthetic oil and/or synthetic liquid products. Numerous definitions of synthetic fuel include fuels produced from biomass as well as industrial and municipal waste. On the one hand, “synthetic” means that the fuel is produced artificially. Unlike synthetic fuel, conventional fuel is usually obtained by separating crude oil into separate fractions (distillation, rectification, etc.) without chemically modifying the components. However, various chemical processes can also be used in the production of traditional fuels. The term “synthetic” can emphasize, on the other hand, that the fuel was produced by chemical synthesis processes, that is, the production of higher-level compounds from several lower compounds. This definition applies in particular to XtL fuels, in which the feedstock is first decomposed into synthesis gas of lower compounds (H2, CO, etc.) in order to obtain higher hydrocarbons (Fischer-Tropsch synthesis). However, even with conventional fuels, chemical processes can be part of the production process. For example, hydrocarbons with carbon chains that are too long can be broken down into shorter chain products, such as those found in gasoline or diesel fuel, through so-called cracking. As a result, depending on the definition, it may not be possible to clearly distinguish conventional from synthetic fuels. Although there is no precise definition, the term "synthetic fuel" is generally limited to XtL fuel. The difference between synthetic and alternative fuels lies in the method of application of the fuel. That is, alternative fuels may require more extensive engine or fuel system modifications, or even the use of a non-traditional engine type (such as steam).
What is hydrogenation
Hydrogenation is a technological process for producing synthetic gasoline from brown coal. The stages are as follows:
- softening coal, mixing with a fatty viscous liquid (fuel oil, tire oil) to obtain a paste-like component;
- placing the paste in an airtight container;
- adding solvent and catalyst to enrich coal.
Under the influence of a temperature of +500 degrees, high pressure of 200 atm, coal turns into a liquid state, then into a vapor state. It is spun in a centrifuge to remove coke and undergoes a distillation and hydrogenation process to obtain the final product.

Gas - Father, Oil - Mother...
Creativity on the topic of the day. I believe in parents!

For those interested: a mug from Ikea, decor – polymer clay, paint for ceramics.
BbI40K
6 years ago
Producing gasoline by gasification
The Fischer-Tropsch or gasification method is step-by-step:
- Coal raw materials are combined with water. Placed in a sealed steam vessel.
- Heats up to t + 350 degrees and is subjected to pressure of 30 atmospheres.
The process produces synthetic gas, which is placed in another sealed vessel filled with a catalyst (iron, cobalt). The fuel comes out and is cracked to produce diesel fuel.
Gasoline can be produced by thermal treatment of coal. Although it is difficult to produce in artisanal conditions. You will need equipment similar to a blast furnace. The method is identical to the pyrolysis process. Coal is placed in a vessel without oxygen, subjected to temperature +200 degrees and a decomposition process with a transition from a solid to a gaseous state.
How to make gasoline from gas
You will need a unit like a mixing vessel to understand how to make fuel from gas. For example, made of stainless steel and metal. Into the vessel:
- Water and propane-butane gas are added.
- Heating occurs to t inside the mixer +120, where mixing of water vapor with gas begins to occur.
Gas already in mixed form:
- fed into a reactor filled with a catalyst made of aluminum and nickel shavings;
- heats up to t+500 g, forming a synthetic substance;
- goes into the refrigerator, cools to t + 35-40 g;
- fed into a sealed container with a catalyst made of zinc and copper shavings;
- is subjected to pressure and t +270 degrees, forming synthetic fuel.
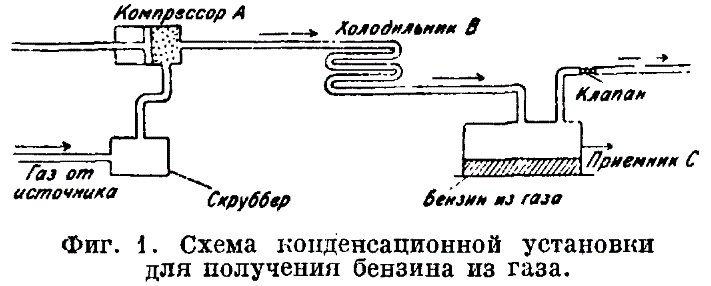
The resulting vapors are transferred from the container to the refrigerator. Subject to cooling and condensation under the influence of condensate. Gas and synthetic gasoline not dissolved in water enter the condenser. A synthetic product is drained from it. The gas can be sent back for recycling.
Liquefied gas

Liquefied petroleum gas (LPG) has been gaining popularity in Europe for several decades, and recently in Russia. Converting a machine to run on propane-butane is not an easy task from the point of view of official registration, but the benefits become more and more obvious every year.
In addition, if gas cylinder equipment (LPG) is installed on the car, you can not only save a lot of money, but also extend the service life of the engine. When gas burns, less soot and soot are released, and the oil becomes much less contaminated.
Making gasoline from tires
Extracting fuel from rubber tires is profitable and exciting. You will need 3 metal barrels with lids, a blast furnace (heat source), a distiller, raw materials (waste).
Stages:
- cut the rubber into small pieces;
- take a fireproof container, connect a heat-resistant tube, immerse the prepared raw materials in it;
- take the end of the tube into the second vessel, which has 2 tubes (for removing gases and receiving liquid fuel);
- fill the third vessel with water as a condenser;
- on the lid of a vessel with two tubes, place the first end 1-2 cm;
- connect the condenser tube to the gas exhaust tube;
- place the second condenser tube under the first vessel and connect it to the gas burner;
- place the tube from the first vessel in a pipe of the largest diameter, because water will flow through it for cooling;
- light the main burner so that water begins to flow into the cooling circuit and the rubber turns into steam.
When passing through the pipe, the gas will cool, flow into the second vessel in the form of condensate, and flow through the outlet tube to the bottom of the condenser.
When the rubber in the first vessel runs out, turn off the water and burner.
Of course, you cannot get high-quality fuel from tires. But for refueling a chainsaw or heating a room, prepared gasoline is quite suitable.

Attention! The method cannot be used in closed rooms, apartments or private houses. In the process, smoke and fumes will rise into the air.
What makes it possible to ensure high heat transfer from smokeless briquettes? And how did you measure it?
Sergey Stepanov:
To measure heat transfer, 10 kilograms of briquettes were burned in a household boiler, and the supply of useful heat (hot water) was determined using a heat meter.
The high efficiency of the boiler is ensured by the fact that the boiler operates evenly most of the time. When burning coal, the combustion mode is different - first there is a peak heat release, then attenuation. Therefore, with coal, most of the heat simply flies away into the chimney.
By the way, when we talk about high heat transfer and fuel economy, it is important to understand that you need to load briquettes in the same volume as you usually load coal. The fact that you need 1.5-2 times less briquettes for heating means that you will have to load fuel 1.5-2 times less often
There is no need to reduce the single portion of loading!
Security measures
- Methanol is the strongest poison. If you accidentally swallow 30 ml, it can be fatal. When working with liquid, it is important to follow safety rules, wear a respirator, and avoid contact with eyes and mouth.
- The process of making ethanol at home is similar to moonshine brewing. Assumes using a torch. Experiments should be carried out away from flammable objects and materials.
- It is unacceptable to use open fire sources when producing fuel from oil.
Of course, producing gasoline at home is difficult. More likely, you will end up with fuel derivatives that will not run the vehicle. To obtain a pure homemade product you will have to work hard. It is important to have not only technical skills and the necessary equipment, but also patience and ingenuity. It is recommended to first read professional literature about the intricacies and nuances of this production. In addition, the business may become unprofitable and even illegal. To produce surrogate fuel in large volumes, finance will be required to purchase expensive equipment and raw materials. The costs will be no less than buying regular gasoline for your car at the nearest gas station.
What is useful for making a coil
To make a heated towel rail, you will need a tube with a diameter of 10-12 mm, a wall thickness of 1 mm, and a length of about one and a half meters. The main parameter is the material - it must be, firstly, non-toxic, and secondly, not react with alcohol-containing products. It must also have good thermal conductivity. The most suitable materials for the coil are copper and silver, brass, aluminum, and stainless steel. The most affordable materials are copper and aluminum, but copper has better heat conductivity.
When selecting a copper tube, remember that sometimes it will need to be cleaned and even replaced. Over time, a black coating forms on the copper surface due to adhering sulfur. To cleanse, you can use the following recipe: tbsp. Dilute a spoonful of citric acid in a liter of warm water. Immerse the heated towel rail in the resulting liquid and wait a little. In most cases, after twenty minutes the plaque is cleaned off quite well. If you can’t completely clean the heated towel rail, add a teaspoon of soda to a liter of water and leave it in this solution overnight, after which everything will come off easily.