Coal is one of the oldest fuels known to man. And even today it occupies a leading position in terms of volume of use. The reason for this is its prevalence, ease of extraction, processing and use. But what is he? What is the chemical formula of coal?
In fact, this question is not entirely correct. Coal is not a substance, it is a mixture of different substances. There are a lot of them, so it is impossible to completely determine the composition of coal. Therefore, by the chemical formula of coal in this article we will rather mean its elemental composition and some other features.
You may be interested in: Speech therapy room: do-it-yourself design
But what can we learn about the state of this substance? Coal is formed from plant remains over many years due to exposure to high temperature and pressure. And since plants are organic in nature, organic substances will predominate in the composition of coal.
Depending on the age and other conditions of origin of coal, it is divided into several types. Each type differs in its elementary composition, the presence of impurities and other important characteristics.
Brown coal
It is the youngest type of coal. It even exhibits a plant-like woody structure. It is formed directly from peat at a depth of about 1 kilometer.
This type of coal contains a fairly large amount of moisture: from 20 to 40%. When exposed to air, it evaporates and the coal crumbles into powder. Next we will talk about the chemical composition of this particular dry residue. The amount of inorganic impurities in brown coal is also large and amounts to 20-45%. These impurities include silicon dioxide, aluminum, calcium and iron oxides. It may also contain alkali metal oxides.
This coal contains a lot of volatile organic and inorganic substances. They can make up up to half the mass of this type of coal. The elemental composition excluding inorganic and volatile substances is as follows:
- Carbon 50-75%.
- Oxygen 26-37%.
- Hydrogen 3-5%.
- Nitrogen 0-2%.
- Sulfur 0.5-3%.
Coal

In terms of formation time, this type of coal comes next after brown coal. It has a black or gray-black color, as well as a resinous, sometimes metallic sheen.
The moisture content of hard coal is significantly less than brown coal: only 1-12%. The content of volatile substances in coal varies greatly depending on the location of mining. It can be minimal (from 2%), but can reach values similar to brown coal (up to 48%). The elemental composition is as follows:
- Carbon 75-92%.
- Hydrogen 2.5-5.7%.
- Oxygen 1.5-15%.
- Nitrogen up to 2.7%.
- Sulfur 0-4%.
From this we can conclude that the chemical formula of hard coal consists of more carbon than that of brown coal. This makes this type of coal a higher quality fuel.
Open mining method
The main advantage of open-pit coal mining is its relative safety. The thing is that it is used only if the depth of the rock is no more than 100 meters. In other words, a shaft is not created that could collapse during an accident. The extraction process itself is carried out according to the following procedure.
First you need to remove the top layer of soil that covers the rock. This layer is called overburden, and the method of removing it is called stripping. This procedure, depending on the type of soil, is carried out using bulldozers, draglines, rotary excavators or scrapers. After the soil layer has been removed, you can proceed to crushing the rock itself. For this, crushers, water cannons, bulldozers and other equipment are used. If the rock in a coal deposit is too dense, then in rare cases, drilling and blasting of coal is used. This mining method usually covers a fairly large area.
As for the disadvantages of the method, they are as follows:
- Firstly, causing significant harm to the environment at the mining site.
- Secondly, all the rock that is mined in this way contains a large amount of harmful impurities in its composition.
The main advantages of open-pit coal mining, in addition to safety, are high speed and cost-effectiveness.

Anthracite

Anthracite is the oldest form of fossil coal. It is characterized by a dark black color and has a characteristic metallic sheen. This is the best coal in terms of the amount of heat it releases during combustion.
The amount of moisture and volatile substances in it is very small. About 5-7% for each indicator. And the elemental composition is characterized by an extremely high carbon content:
- Carbon over 90%.
- Hydrogen 1-3%.
- Oxygen 1-1.5%.
- Nitrogen 1-1.5%.
- Sulfur up to 0.8%.
More coal is contained only in graphite, which is a further stage of anthracite coalification.
Charcoal

This type of coal is not a fossil, so it has some peculiarities in its composition. It is produced by heating dry wood to a temperature of 450-500 oC without air access. This process is called pyrolysis. During this process, a number of substances are released from the wood: methanol, acetone, acetic acid and others, after which it turns into coal. By the way, burning wood is also pyrolysis, but due to the presence of oxygen in the air, the released gases ignite. This is what determines the presence of flames during combustion.
Wood is not homogeneous; it has a lot of pores and capillaries. A similar structure is partially preserved in the coal obtained from it. For this reason, it has good adsorption capacity and is used along with activated carbon.
The humidity of this type of coal is very small (about 3%), but during long-term storage it absorbs moisture from the air and the percentage of water increases to 7-15%. The content of inorganic impurities and volatile substances is regulated by GOSTs and should be no more than 3% and 20%, respectively. The elemental composition depends on the production technology, and looks approximately like this:
- Carbon 80-92%.
- Oxygen 5-15%.
- Hydrogen 4-5%.
- Nitrogen ~0%.
- Sulfur ~0%.
The chemical formula of charcoal shows that in terms of carbon content it is close to stone, but in addition it has only a small amount of elements unnecessary for combustion (sulfur and nitrogen).
Maximum coal combustion temperature (video)
Today, this use of a variety of solid fuels, in the form of wood, coal or peat, is popular. It is used not only in everyday life for heating or cooking, but in many industries.
Comments
0 Daniil 02/16/2018 13:06 I never thought about combustion temperature, but in practice anthracite showed itself to be the best.
It burns longer and leaves very little buzz after it, unlike regular coal. As a result, anthracite is more economical, burns well and leaves little waste after it. Quote
Update list of comments RSS feed of comments for this entry
Activated carbon
Activated carbon is a type of carbon with a high specific pore surface area, which makes it even more absorbent than wood. The raw materials used for its production are charcoal and coal, as well as coconut shells. The starting material is subjected to an activation process. Its essence is to open clogged pores using high temperature, electrolyte solutions or water vapor.
During the activation process, only the structure of the substance changes, therefore the chemical formula of activated carbon is identical to the composition of the raw materials from which it was made. The moisture content of activated carbon depends on the specific pore surface area and is usually less than 12%.
Source
Carbon
Carbon
Carbon is a non-metallic element of group IV of the periodic table D.I. Mendeleev, is the most important part of all organic substances in nature.

General characteristics of group IVa elements
From C to Pb (from top to bottom in the periodic table) there is an increase in: atomic radius, metallic, basic, reducing properties. Electronegativity, ionization energy, and electron affinity decrease.
Of the elements of group IVa, carbon and silicon are non-metals, germanium, tin and lead are metals.
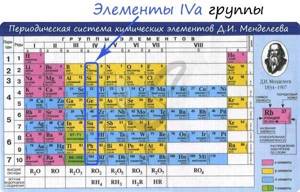
The electronic configurations of these elements are similar, since they are in the same group (main subgroup!), the general formula is ns2np2:
- C - 2s22p2
- Si - 3s23p2
- Ge - 4s24p2
- Sn - 5s25p2
- Pb - 6s26p2
Natural compounds
In nature, carbon occurs in the form of the following compounds:
- Allotropic modifications - graphite, diamond, fullerene
- MgCO3 - magnesite
- CaCO3 - calcite (chalk, marble)
- CaCO3*MgCO3 - dolomite

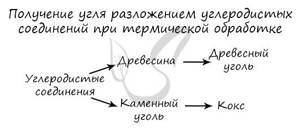
Receipt
Carbon is obtained during the pyrolysis of hydrocarbons (pyrolysis - heating without access to oxygen). The production of carbon compounds: wood and coal is also used.
C2H6 → (t)C + H2 (ethane pyrolysis)
Chemical properties
- Reactions with nonmetals
- Reactions with metals
- Restorative properties
- Reaction with water
- Reactions with acids
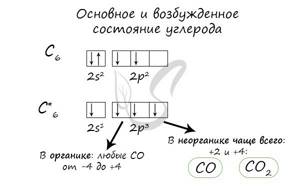
When heated, carbon reacts with many non-metals: hydrogen, oxygen, fluorine.
C + H2 → (t) CH4 (methane)
2C + O2 → (t) 2CO (carbon monoxide is a product of incomplete oxidation of carbon, formed when there is a lack of oxygen)
C + O2 → (t) CO2 (carbon dioxide is a product of complete oxidation of carbon, formed when there is a sufficient amount of oxygen)
C + F2 → (t)CF4
When heated, carbon reacts with metals, exhibiting its oxidizing properties. Let me remind you that metals can only take positive oxidation states.
Ca + C → CaC2 (calcium carbide, CO carbon = -1)
Al + C → Al4C3 (aluminum carbide, CO carbon -4)
Obviously, the degree of oxidation of carbon in combination with different metals may differ.

Carbon is a good reducing agent. With its help, the metallurgical industry copes with the task of obtaining pure metals from their oxides:
Fe2O3 + C → Fe + CO2
ZnO + C → Zn + CO
FeO + C → Fe + CO
Carbon reduces not only metals from their oxides, but also nonmetals in a similar way:
SiO2 + C → (t) Si + CO
It can also reduce its own oxide:
CO2 + C → CO

The well-known reaction of interaction of coal with water vapor, also called gasification of coal, peat, shale, is extremely important in industry:
C + H2O → CO↑ + H2↑
In reactions with acids, carbon acts as a reducing agent:
C + HNO3(conc.) → (t) CO2 + NO2 + H2O
C + HNO3 → CO2 + NO + H2O
C + H2SO4(conc.) → CO2 + SO2 + H2O

Carbon monoxide II - CO
Carbon monoxide II is a product of incomplete oxidation of carbon. Non-salt-forming oxide. This extremely dangerous substance is often formed during fires in confined spaces, or when a car is warmed up in a garage.
Dissolving in the blood, carbon monoxide (which has a 300 times greater affinity for hemoglobin than oxygen) easily outcompetes oxygen and takes its place in red blood cells. Carbon monoxide poisoning is often fatal.
Receipt
In industry, carbon monoxide is produced by the reduction of carbon monoxide IV or gasification of coal (t = 1000 °C).
CO2 + C → (t) CO
C + H2O → (t) CO + H2
In the laboratory, carbon monoxide is obtained from the decomposition of formic acid in the presence of sulfuric acid:
HCOOH → (H2SO4)CO + H2O
Chemical properties
Completely oxidizes to carbon dioxide in reaction with oxygen, reducing metal oxides.
CO + O2 → CO2
Fe2O3 + CO → Fe + CO2
FeO + CO → Fe + CO2
Formation of carbonyls - extremely toxic substances.
Fe + CO → (t) Fe(CO)5

Carbon monoxide IV - CO2
Product of complete oxidation of carbon. Refers to acidic oxides, corresponds to carbonic acid H2CO3. Colorless gas, odorless.
Receipt
In industry, carbon dioxide is obtained from the decomposition of limestone, during the production of alcohol, and from the alcoholic fermentation of glucose.
CaCO3 → (t) CaO + CO2↑
C6H12O6 → C2H5OH + CO2↑
In laboratory conditions, the reaction of chalk (marble) with hydrochloric acid is used.
CaCO3 + HCl → CaCl2 + H2O + CO2↑
Carbon dioxide is formed when organic substances burn:
C3H8 + O2 → CO2 + H2O

Chemical properties
- Reaction with water
- Reactions with basic oxides and bases
- Oxidative properties
As a result of the reaction with water, unstable carbonic acid is formed, which immediately breaks down into water and carbon dioxide.
CO2 + H2O ⇄ H2CO3
During reactions with bases and basic oxides, carbon dioxide forms salts of carbonic acid: medium - carbonates (with an excess of base), acidic - bicarbonates (with an excess of acidic oxide).
2KOH + CO2 → K2CO3 + H2O (base-acid oxide ratio 2:1)
KOH + CO2 → KHCO3 (base-acid oxide ratio 1:1)
Na2O + CO2 → Na2CO3
When heated, it is capable of oxidizing metals to their oxides.
Zn + CO2 → (t) ZnO + CO

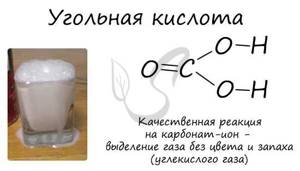
Carbonic acid
A weak dibasic acid, existing only in solutions, decomposes into water and carbon dioxide.
Chemical properties
- Qualitative reaction
- Medium and acidic salts
- Heating carbonic acid salts
The presence of a carbonate ion can be determined using an acid: this reaction is accompanied by “boiling” - the appearance of bubbles of a colorless, odorless gas.
MgCO3 + HCl → MgCl2 + CO2↑ + H2O
I have more than once encountered a description of the reactions associated with this acid, which deserves our attention. The assignment said that when carbon dioxide was added to a solution of calcium hydroxide, a precipitate appeared, and with further passage of carbon dioxide, the turbidity disappeared.
This can be easily explained by remembering the ability of carbonic acid to form acid salts that are soluble.
Ca(OH)2 + CO2 → CaCO3 (precipitate forms)
CaCO3 + H2O + CO2 → Ca(HCO3)2 (precipitate dissolves)

To make an acid salt (bicarbonate) from a medium salt (carbonate), you need to add carbonic acid. However, writing its formula H2CO3 is a mistake. It should be written as water and carbon dioxide.
Li2CO3 + CO2 + H2O → LiHCO3 (average salt + acid = acid salt)
To return the average salt, you should add alkali to the sour salt.
LiHCO3 + LiOH → Li2CO3 + H2O
When heated, carbonates decompose into the corresponding metal oxide and carbon dioxide, and bicarbonates into metal carbonate, carbon dioxide and water.
MgCO3 → (t) MgO + CO2
KHCO3 → (t) K2CO3 + CO2↑ + H2O
© Bellevich Yuri Sergeevich 2018-2021
This article was written by Yuri Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain article materials and permission to use them, please contact Yuri Bellevich
.