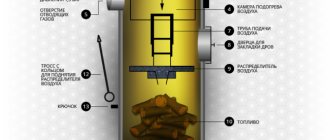
Rice. 17. Design and main elements of a cast iron boiler
During operation, cast iron becomes covered with dry rust, which is a film of iron oxide (this is the so-called chemical corrosion). As a rule, dry rust does not progress.
Cast iron undergoes wet corrosion much more slowly than steel. Cast iron heat exchangers can be cleaned less frequently - their efficiency, even due to the appearance of carbon deposits during operation, decreases less.
A minor disadvantage of a cast iron heat exchanger is the possibility of thermal shock: if cold water enters a cast iron heat exchanger that has not cooled down, it may crack, so large temperature differences between the supply and return lines should be avoided. The thermal inertia of cast iron boilers is higher; they take a long time to heat up, but on the other hand, they cool down more slowly.
The steel boiler is a one-piece monoblock, which is assembled and welded in a factory.
Boilers made of steel are less susceptible to destruction from sudden temperature changes. Steel is more elastic than cast iron and can easily withstand temperature differences, even if the boiler is fed with cold water into the return line. The ability of steel to easily withstand temperature changes allows solid fuel boilers with a steel heat exchanger to more widely use boiler automation. Steel boilers react faster than cast iron boilers, they cool faster and heat up faster.
However, we should not forget that with large and frequent temperature changes, “fatigue zones” may appear in steel and, as a result, cracks in places weakened by welding. If a steel boiler is rusted or damaged by deposits of hardness salts, then repair will most likely be simply impossible - getting factory-quality welding at home is almost impossible. In fact, a burnt-out steel boiler will have to be thrown away, whereas in a cast-iron boiler the repairman will simply replace the damaged section.
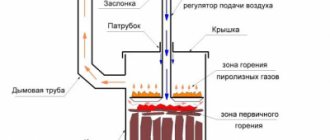
Traditional solid fuel boilers (Fig. 18) in their design resemble a conventional stove with a window for fuel supply, a firebox and a chimney. The fuel for a traditional solid fuel boiler can be either coal or wood. The main element of a traditional solid fuel boiler is a heat exchanger, which ensures the transfer of thermal energy to the coolant.
The obvious advantage of a traditional low-power solid fuel boiler, suitable for individual heat supply, is the absence of electronic boards, automation and all kinds of control systems, which are the first to fail. The only automation device is a temperature controller that operates on a mechanical principle, so a traditional solid fuel boiler is not only quite versatile, but also reliable, capable of operating for a long time without maintenance.
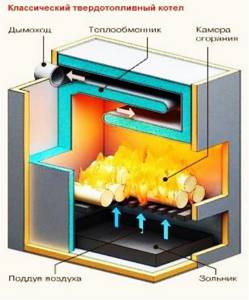
Rice. 18. Traditional solid fuel boiler design
Pyrolysis boilers produce thermal energy by burning solid fuel. A pyrolysis boiler has a higher efficiency, and, therefore, more thermal energy can be obtained from a smaller volume of wood than with the same operation of a traditional boiler.
Wood combustion occurs in three phases according to the principle of gas formation when burning wood:
1 - drying firewood;
2 - degassing (approximately 85% of substances during the combustion of wood turn into flammable gases, the rest remain charcoal);
3 - combustion - at temperatures above 600 °C, combustible gases oxidize and ignite and a burning layer of charcoal is formed. From approximately 900 oC to 1000 oC, thermal decomposition of charcoal and oxidation of carbon in it occurs.
The fan in the boiler directs the flame downward, making the entire fuel combustion process controllable. In addition, a constant supply of oxygen to the combustion zone guarantees complete oxidation of combustible gases. For this purpose, in addition to the main air, preheated secondary air is supplied for post-combustion.
Long-burning boilers (top-burning boilers) are a type of solid fuel boiler in which the air supply and combustion process are limited to the upper part of the fuel layer. This scheme allows you to simultaneously load a significant amount of fuel into the firebox. Such boilers are characterized as long-burning boilers and require less frequent maintenance.
A pellet boiler is a solid fuel boiler that uses wood pellets as fuel. Wood pellets are made from wood waste: shavings, sawdust and other residues from the woodworking industry.
At its core, a pellet boiler is not much different from a traditional solid fuel boiler in which wood is burned. The main difference is that the pellet boiler, in addition to the combustion chamber, is equipped with a special hopper and automatic fuel supply (Fig. 19-20).
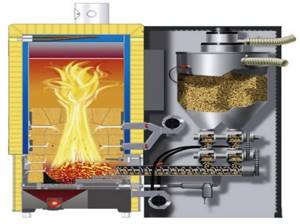
Rice. 19. Boiler with fuel bunker and automatic fuel supply

Rice. 20. Boilers with a fuel bunker and automatic fuel supply

Rice. 21. Boilers with a fuel bunker and automatic fuel supply
Fig. 22. Boiler with a fuel bunker and automatic fuel supply
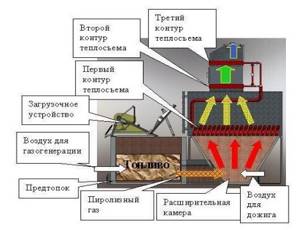
Rice. 23. Diagram of the boiler and the processes occurring in it
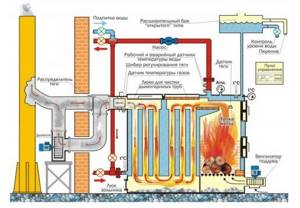
Rice. 24. Construction of a boiler unit for burning wood
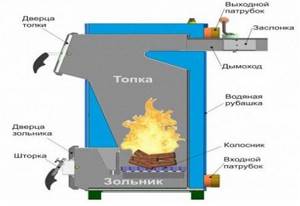
Rice. 25. Construction of a boiler for burning wood
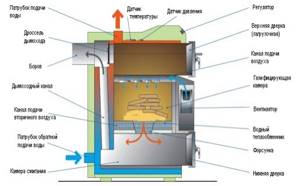
Rice. 26. Construction of a boiler for burning wood
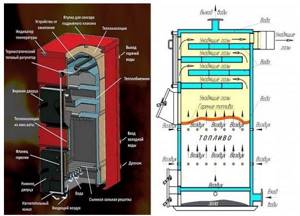
Rice. 27. Construction of a boiler for burning solid fuel
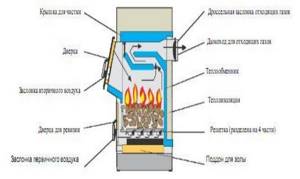
Figure 28. Construction of boilers for burning solid fuels
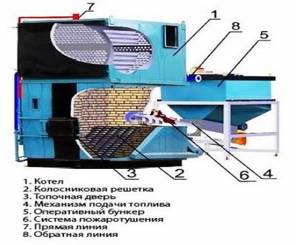
Rice. 29. Construction of boilers for burning solid fuels
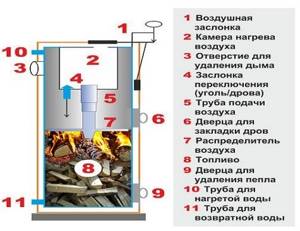
Rice. 30. Construction of boilers for burning solid fuels
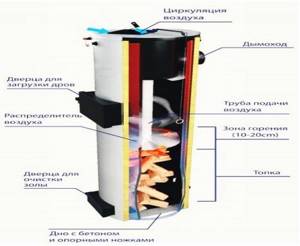
Rice. 31. Construction of boilers for burning wood
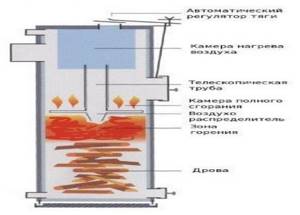
Rice. 32. Construction of a boiler for burning wood
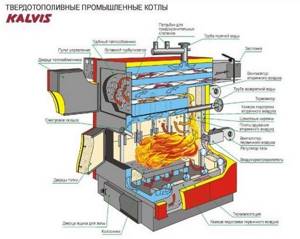
Rice. 33. Solid fuel industrial boiler
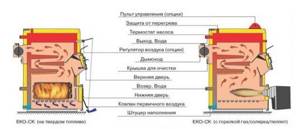
Rice. 34. Boilers for working on different types of fuel (solid, liquid, gas)
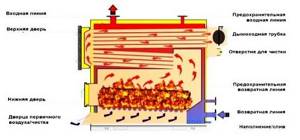
Rice. 35. Construction of a boiler for burning solid fuel
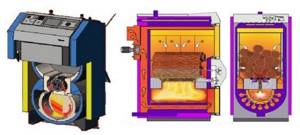
Rice. 36. Construction of a boiler for burning wood (three types of boiler)

Rice. 37. Construction of long-burning wood-burning boilers
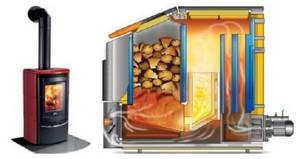
Rice. 38. Construction of a long-burning wood-burning boiler (two types)
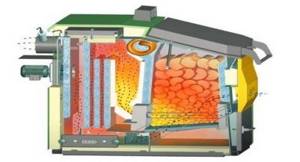
Rice. 39. Construction of a long-burning wood-burning boiler (two types)
Boilers for burning liquid fuels and combustion in them
Liquid fuel boilers are structurally somewhat different from solid fuel boilers.
For universal boilers designed to burn liquid fuel, a special burner with a safety circuit breaker is supplied, without which the operation of the boilers is not allowed.
Combination boilers for heating a house can use not one, but several types of fuel. For this feature they are also called universal. Their main advantage is the ability to work during interruptions in the main type of fuel.
Depending on the fuel combinations, there are several types of boilers, which differ in design and type of burner.
A feature of liquid-fuel heating boilers is the use of fuel oil, diesel fuel or kerosene as fuel. The main chemical elements that make up any liquid fuel for heating boilers are oxygen, hydrogen, carbon and sulfur. Due to the presence of non-combustible minerals and moisture in fuel oil, ash is formed during the combustion of liquid fuel.
The ash content of fuel oils is mainly determined by the content of oxygen-containing compounds containing metal cations. Some ash comes from suspended particles (mainly silicates and silica). When moving to more viscous fuel oils, the content of suspended particles and colloidal particles increases. Ash is an extremely undesirable component of fuel oil combustion products, as it clogs nozzles, accelerates corrosion of equipment and requires periodic shutdown and cleaning of boiler equipment. The most dangerous are vanadium compounds (in ash they are represented by vanadium pentoxide V2O5), which sharply reduce the resistance of most steels to high-temperature corrosion.
The main properties of boiler oil as a liquid fuel, which determine its use in boiler plants, are: sulfur content, viscosity, pour point, ash content, lower calorific value, density.
Fuel oil is a mixture of hydrocarbons (with a molecular weight of 400 to 1000), petroleum resins (with a molecular weight of 500-3000 or more), asphaltenes, carbenes, carboids and organic compounds containing metals (V, Ni, Fe, Mg, Na, Ca). The physicochemical properties of fuel oil depend on the chemical composition of the source oil and the degree of distillate fraction distillation and are characterized by the following data: viscosity 8-80 mm2/s (at 100 °C), density 0.89-1 g/cm3 (at 20 °C) , pour point 10-40 °C, sulfur content 0.5-3.5%, ash up to 0.3%, lower calorific value 39.4-40.7 MJ/mol.
Based on the amount of sulfur, fuel oils are divided into low-sulfur, sulfur and high-sulfur with a sulfur content of up to 0.5%, 2% and 3.5%, respectively. The sulfur content in fuel oil depends on the source oil, but is significantly higher than in it, since sulfur is concentrated mainly in heavy residual products. When processing high-sulfur oils, the sulfur content in fuel oil can reach up to 4.3%. Sulfur is contained in fuel oils in active and passive forms. Active sulfur causes corrosion of pipelines, tank heaters, heat exchangers and tail heating surfaces at metal wall temperatures below the dew point temperature.
Fuel oils from oil refineries usually contain only traces of water. Fuel watering occurs during transportation and especially when heated with open steam. When heating fuel oil with open steam, the moisture content in the fuel oil increases sharply, which not only causes losses of steam and condensate, but also reduces the quality of the fuel oil itself. As a result, the efficiency of the boiler units and the reliability of the boiler room operation decreases. When heated in open tanks, the water contained in the fuel oil causes foaming.
Boiler fuel oil can be low-viscosity or high-viscosity with a high content of resinous substances and paraffin. The viscosity of fuel oil is an important operational factor that determines the ability to transport, drain, pump and burn it. With increasing temperature, the viscosity of fuel oil decreases, therefore all operations with fuel oil are carried out with heating.
Depending on the viscosity, boiler fuel oil comes in several grades, differing in their pour point, which is always above 0 °C. For the most viscous grades of fuel oil, the pour point is 25 °C and higher, so preheating of such fuel oil is necessary: when pumping up to 60-70 °C, and when burning up to 140 °C.
The flash point of fuel oil is the temperature at which its vapor forms a mixture with the surrounding air that ignites when a fire is brought to it.
When heating fuel oil in open (without pressure) containers, for fire safety purposes, the heating temperature should be approximately 10 °C below the flash point. In closed containers (coils, pipes) under pressure, fuel can be heated to fuel oil significantly above its flash point.
Currently, gas-oil burners of various designs are installed on water-tube boilers (DE, DKVR) and water heating units (KV-GM), which meet the requirements of economical and safe operation. The main thing is to ensure approximately equal combustion quality and flame length for both types of fuel (natural gas and fuel oil). Gas-oil burners are a complex of a gas burner and an oil nozzle and, depending on the design, are designed for separate or joint combustion of gas and liquid fuels. To install the burner in the front wall (lining) of the boiler, an embrasure is made.
In decentralized heat supply, as a rule, diesel fuel and light grades of fuel oil are used. First of all, this is due to the convenience of their transportation and storage, low viscosity, which facilitates the task of efficient combustion, as well as low sulfur and ash content, which solves the problem of environmental pollution and equipment safety.
Diesel boilers are boilers that run on diesel fuel, and it is always possible to replace a diesel burner with a gas burner (thanks to this, these boilers can be considered universal). Burners for diesel boilers are selected based on the power of the boiler. Diesel boilers are the optimal solution when gas has not yet been supplied, but it is planned to be supplied. In this case, you will not need to buy another boiler or change the heating and hot water supply system, but simply change the burner to a gas one.

Rice. 40. Construction of a boiler for burning liquid fuel
Rice. 41. Construction of boilers for burning liquid fuel
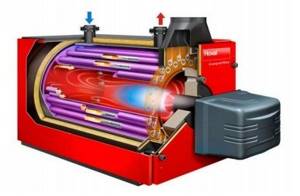
Figure 42. Construction of boilers for burning liquid fuel

Rice. 43. Construction of a boiler for burning liquid fuel (two types)
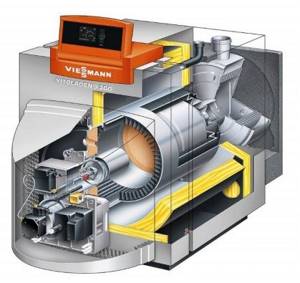
Rice. 44. Construction of boilers for burning liquid fuel
Boiler classification
According to the nature (type) of the generated coolant:
- steam,
- hot water,
- steam-water-heating.
According to coolant parameters:
- steam boilers with a working steam pressure pp of more than 0.7 kgf/cm2 and hot water boilers with a water heating temperature tb above 115 °C are subject to boiler inspection (Rostechnadzor of Russia); they are subject to PB 10-574–03 “Rules for the design and safe operation of steam and hot water boilers” - “supervisory” boilers;
- steam boilers with a working steam pressure pp of no more than 0.7 kgf/cm2 and hot water boilers with a water heating temperature tw of no higher than 115 °C are “non-supervisory” boilers; they are subject to the “Rules for the design and safe operation of steam boilers with a steam pressure of not more than 0.07 MPa (0.7 kgf/cm2), hot water boilers and water heaters with a water heating temperature not exceeding 388 K (115 °C)”, approved Ministry of Construction of Russia.
According to the material used:
- steel,
- cast iron
According to the principle of heat exchange:
- surface (recuperative), in which heat transfer from combustion products to boiler water occurs through a dividing wall (heating surface);
- contact, in which heat transfer occurs through direct contact (mixing) of gases and water.
Based on the movement of combustion products and water, surface boilers are divided into:
- water-tube, in which boiler water moves through pipes, and combustion products move outside the pipes;
- gas-tube (with a flame tube (furnace) and a bundle of smoke tubes), in which combustion products move inside pipes washed externally with water;
- water-tube-gas-tube (boiler VK-32, in which the combustion part is water-tube, and the convective part is gas-tube).
On the organization of the combustion process (method of air supply and removal of combustion products):
- working under vacuum; can have draft and air supply natural or forced. In the gas path (and in the furnace) the pressure is below atmospheric (draft);
- operating with back pressure (supercharged); the firebox and flues are under excess pressure relative to the surrounding air. Air supply and combustion products removal are carried out forcibly from a blower fan.
According to the nature of the movement of boiler water (steam-water mixture):
- with natural circulation (Fig. 1, a);
- with forced circulation, when water moves due to the pressure created by pumps: with multiple forced circulation (Fig. 1, b; not widely used);
- straight-through (without drums). Large power boilers at thermal power plants operate according to this scheme (Dp = 3950 t/h, rp = 255 kgf/cm2, tpp = 560 °C) and almost all hot water boilers (Fig. 1, c).
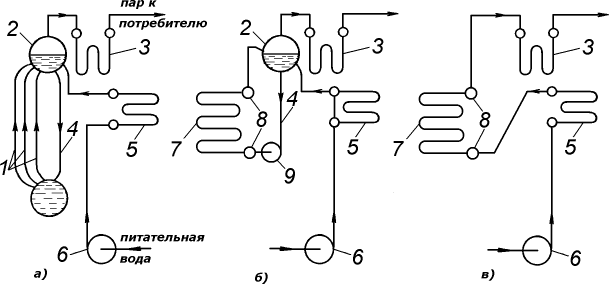
Rice. 1. Water circulation patterns in steam boilers: a – natural; b – forced multiple; c – forced direct flow; 1 – evaporative rise pipes; 2 – upper drum of the boiler; 3 – steam superheater; 4 – lowering pipes; 5 – water economizer; 6 – feed pump; 7 – evaporation pipes; 8 – collectors; 9 – incentive circulation pump
Boilers for burning gas and combustion in them
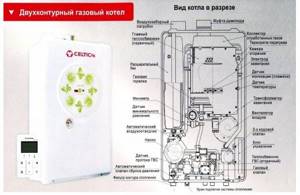
Rice. 45. Construction of boilers for burning gaseous fuels

Rice. 46. Construction of boilers for burning gaseous fuels
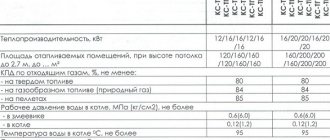
Main technical characteristics of steam and hot water boilers
The design parameters characterizing the operation of the boiler are indicated in the boiler passport, drawn up by the manufacturer in the prescribed form (Appendix 4 to PB 10-574–03) and kept by the owner for the entire period of operation.
Each boiler must be affixed with a nameplate containing the nameplate data, printed in a manner that ensures clarity and durability of the image.
The following data must be written on the steam boiler plate:
- name, trademark of the manufacturer;
- boiler designation;
- boiler number according to the numbering system of the manufacturer;
- Year of manufacture;
- nominal steam output Dp in t/h;
- working pressure at the outlet in MPa (kgf/cm2);
- nominal steam outlet temperature in °C.
The following data must be written on the boiler plate:
- name, trademark of the manufacturer;
- boiler designation;
- boiler number according to the numbering system of the manufacturer;
- Year of manufacture;
- nominal heating capacity Q in MW (Gcal/h); working pressure at the outlet in MPa (kgf/cm2);
- nominal outlet water temperature in °C.
The designation of the steam boiler includes:
- type,
- steam capacity (t/h),
- absolute (excess) steam pressure pp, (MPa or kgf/cm2),
- type of fuel (G – gas, M – fuel oil);
- Pressurized boilers are designated by the letter N.
For example: DKVR-10/13; E-25-2.4 GM; DE-6.5/14-225 GM; E-1/9-G.
The designation of the hot water boiler includes:
- type – KV (hot water boiler);
- type of fuel (G – gas, M (F) – fuel oil, diesel fuel);
- type of firebox (H – pressurized);
- rated thermal power (MW or Gcal/h);
- nominal water temperature at the boiler outlet, °C;
- gas pressure (Gn – low; Gs – average);
- an automated boiler is designated by the letter “a”;
- C – steel.
For example: KV-GM-10-50; KSVa-2.5-Gs; KVA-3-95; KVA-0.75Zh-115.
On each boiler put into operation and after technical inspections have been carried out, a plate with a format of at least 300×200 mm indicating the following data must be attached in a visible place:
- registration number;
- permitted pressure;
- date, month and year of the next internal inspection and hydraulic test.
Main technical characteristics of steam boilers:
- rated steam output, Dp, t/h – the maximum working amount of steam produced by the boiler for 1 hour;
- parameters of the resulting steam:
- operating (calculated or permitted) steam pressure, rp, MPa (kgf/cm2);
- test pressure, rtest, MPa (kgf/cm2);
- type of steam (saturated, superheated);
- saturated steam temperature, tsat, °C (at operating steam pressure pp or superheated steam temperature, tpp, °C);
- feed water temperature, °C;
- steam and water volume of the boiler, m3;
- volume of water, m3;
- evaporation time of this volume, min.
Main technical characteristics of hot water boilers:
rated heating capacity (thermal power), Q, Gcal/hour (MW) – the maximum working amount of heat absorbed by water for 1 hour of operation; 1 Gcal/h = 1.163 MW;
water parameters:
- working water pressure, MPa (kgf/cm2);
- minimum permissible water pressure pv at nominal temperature tb;
- test pressure, rtest, MPa (kgf/cm2);
- minimum permissible water temperature at the boiler inlet, °C;
- nominal water temperature at the boiler outlet, °C;
- nominal water flow through the boiler, Gw, m3/h, as well as the minimum and maximum permissible;
- hydraulic resistance, no more than, MPa.
General parameters characterizing steam and hot water boilers:
- type of fuel and its characteristics;
- burner type;
- boiler heating surface: radiation, convective, total, S, m2;
- calculated efficiency, gross, % when burning gas and fuel oil;
- resistance of gas and air paths, Pa (mm water column);
- temperature of combustion products at the exit from the furnace, behind the boiler, temperature of flue gases - when burning gas and fuel oil;
- content of O2, CO, NOX in flue gases;
- design indicators: internal diameter of the drums, drum wall thickness, length of the cylindrical part of the upper and lower drums; diameters of downcomers, screen and convective pipes; pitch of screen pipes, their number; boiler dimensions.
Fuel combustion process
Select hot water boilers in the factory catalog
Price from 220,000
Combustion is a chemical combination of combustible fuel substances with oxygen in the air, accompanied by a sharp increase in temperature and the release of a significant amount of heat. When fuel burns, gaseous products (flue gases) and focal residues in the form of ash and slag are formed. Conventionally, the process of burning solid fuel is divided into three stages:
- ignition (ignition),
- active combustion
- afterburning.

In the first stage, solid fuel is first heated and dried and at a temperature of 105 - 110 ° C loses its moisture. Then, at a temperature of 300 - 400 °C, it begins to decompose into volatile substances and a solid residue. With further heating, when its temperature becomes equal to the ignition temperature, the fuel ignites. The ignition temperature (approximate) of various fuels is as follows, °C: firewood - 300; brown coal 300 - 400; coal 450 - 500; anthracite 700 - 750; liquid fuel 500 - 600; gas is about 600. The stage of active combustion is characterized by high temperature (more than 1000 ° C) with maximum heat release and the greatest consumption of air (oxygen) spent on the combustion of coke and volatile substances.
Afterburning of solid fuel is characterized by decreasing heat release and decreasing air demand.
The combustion of liquid fuel occurs mainly in a vapor-gas environment, when, as a result of preheating, it passes from a liquid state to a vapor state. Since the boiling point of liquid fuel is much lower than its ignition temperature, it first evaporates and then ignites (first the light fractions, then the heavy ones). The rate of fuel evaporation depends on the evaporation area and the amount of heat supplied. The evaporation rate increases sharply when fuel is sprayed into individual droplets using special devices - nozzles.
Combustion of solid fuel (heterogeneous combustion)
To burn fuel, you need a large amount of air, several times the weight of the fuel. When blowing a layer of fuel with air, the aerodynamic pressure force of the flow P can be less than the weight of a piece of fuel G or, conversely, greater than it. In furnaces with a “fluidized bed”, “boiling” is associated with the separation of fuel particles, which increases the volume of the bed by 1.5-2.5 times. The movement of fuel particles (usually from 2 to 12 mm) is similar to the movement of a boiling liquid, which is why such a layer is called “boiling”.
In furnaces with a “fluidized” bed, the gas-air flow does not circulate in the layer zone, but blows through the layer directly. The air flow penetrating the layer experiences non-uniform braking, which creates a complex velocity field in which the particles constantly change their windage depending on their position in the flow. In this case, the particles acquire a rotational-pulsating motion, which creates the impression of a boiling liquid.
The combustion process of solid fuel can be conditionally divided into stages that overlap one another. These stages take place under different temperature and thermal conditions and require different amounts of oxidizing agent.
Fresh fuel entering the firebox undergoes more or less rapid heating, moisture evaporates from it and volatile substances are released - products of dry distillation of fuel. At the same time, the coke formation process occurs. The coke burns and is partially gasified on the grate, and the gaseous products are burned in the combustion chamber. The non-combustible mineral part of the fuel turns into slag and ash during fuel combustion.
Gaseous fuel
Gaseous fuel is a mixture of various gases: methane, ethylene and other hydrocarbons, carbon monoxide, carbon dioxide or carbon dioxide, nitrogen, hydrogen, hydrogen sulfide, oxygen and other gases, as well as water vapor.
Methane (CH4) is the main constituent of many natural gases. Its content in natural gases reaches 93...98%. When 1 m3 of methane is burned, ~35,800 kJ of heat is released.
Gaseous fuels may also contain small amounts of ethylene (C2H4). The combustion of 1 m3 of ethylene produces ~59,000 kJ of heat.
In addition to methane and ethylene, gaseous fuel also contains hydrocarbon compounds, for example propane (C3H8), butane (C4H10), etc. The combustion of these hydrocarbons releases more heat than the combustion of ethylene, but in combustible gases their amount is insignificant.
Hydrogen (H2) is 14.5 times lighter than air. When 1 m3 of hydrogen is burned, ~10,800 kJ of heat is released. Many flammable gases, except coke gas, contain relatively small amounts of hydrogen. In coke oven gas its content can reach 50...60%.
Carbon monoxide (CO) is the main flammable component of blast furnace gas. When 1 m3 of this gas is burned, ~12,770 kJ of heat is generated. This gas is colorless, odorless and very poisonous.
Hydrogen sulfide (H2S) is a heavy gas with an unpleasant odor and is highly toxic. The presence of hydrogen sulfide in the gas increases corrosion of the metal parts of the furnace and gas pipeline. The harmful effects of hydrogen sulfide are enhanced by the presence of oxygen and moisture in the gas. When 1 m3 of hydrogen sulfide is burned, ~23,400 kJ of heat is released.
The remaining gases: CO2, N2, O2 and water vapor are ballast components, since with an increase in the content of these gases in the fuel, the content of its combustible components decreases. Their presence leads to a decrease in the combustion temperature of the fuel. The content of free oxygen in gaseous fuel >0.5% is considered dangerous according to safety regulations.
Lower and upper explosive limits of flammable gases
Another important feature of the combustion of gas-air mixtures is the presence of concentration limits. Combustible gases can ignite or explode if they are mixed in certain (for each gas) proportions with air and heated not lower than their ignition temperature. Ignition and further spontaneous combustion of a gas-air mixture at certain ratios of gas and air is possible in the presence of a fire source (even a spark).
There are lower and upper concentration limits of explosiveness (flammability) - the minimum and maximum percentage of gas in a mixture at which ignition and explosion can occur.
The lower limit corresponds to the minimum, and the upper limit to the maximum amount of gas in the mixture, at which their ignition (during ignition) and spontaneous (without the flow of heat from the outside) propagation of the flame (spontaneous ignition) occur. The same limits correspond to the explosiveness conditions of gas-air mixtures.
The lower explosive limit corresponds to the minimum concentration of fuel vapor in a mixture with air at which a flash occurs when a flame is applied. The upper explosive limit corresponds to the maximum concentration of fuel vapor in a mixture with air, above which flashes no longer occur due to a lack of oxygen in the air. The wider the range of flammability limits (also called explosive limits) and the lower the lower limit, the more explosive the gas is. Most hydrocarbons have low explosive limits. For CH4 methane, the lower and upper explosive limits are 5% and 15% by volume, respectively.
A number of gases have the widest limits of explosion (flammability): hydrogen (4.0 - 75%), acetylene (2.0 - 81%) and carbon monoxide (12.5 - 75%). The volumetric content of flammable gas in a gas-air mixture, below which a flame cannot spontaneously spread in this mixture when a source of high temperature is introduced into it, is called the lower concentration limit of ignition (flame propagation) or the lower explosive limit of a given gas. Thus, a mixture of gas and air is explosive only if the content of flammable gas in it is in the range between the lower and upper explosive limits.
If the gas content in a gas-air mixture is less than the lower flammability limit, then such a mixture cannot burn and explode, since the heat released near the ignition source is not enough to heat the mixture to the ignition temperature.
When the gas content in the mixture is between the lower and upper explosive limits, the ignited mixture ignites and burns both near the ignition source and when it is removed. This mixture is explosive. And if the gas content in the mixture is above the upper explosive limit, then the amount of air in it is not enough for complete combustion of the gas.
The existence of flammability (explosivity) limits is caused by heat losses during combustion. When the flammable mixture is diluted with air, oxygen or gas, heat losses increase, the speed of flame propagation decreases and combustion stops after the ignition source is removed.
As the temperature of the mixture increases, the flammability limits expand, and at temperatures above the auto-ignition temperature, mixtures of gas with air or oxygen burn at any volume ratio.
The flammability (explosivity) limits depend not only on the types of combustible gases, but also on the experimental conditions (vessel capacity, thermal power of the ignition source, mixture temperature, flame propagation up, down, horizontally, etc.). This explains the slightly different values of these limits in various literary sources. When the flame spreads from top to bottom or horizontally, the lower limits increase slightly, and the upper limits decrease.
The calculated excess pressure during the explosion of such mixtures is as follows: natural gas - 0.75 MPa, propane and butane - 0.86 MPa, hydrogen - 0.74 MPa, acetylene - 1.03 MPa. In real conditions, the explosion temperature does not reach maximum values and the resulting pressures are lower than those indicated, but they are quite sufficient to destroy not only the lining of boilers and buildings, but also metal containers if an explosion occurs in them.
The main reason for the formation of explosive gas-air mixtures is gas leakage from gas supply systems and its individual elements (leak closure of valves, wear of stuffing box seals, ruptures of gas pipeline seams, leakage of threaded connections, etc.), as well as imperfect ventilation of rooms, fireboxes and gas ducts boilers and furnaces, basements and various wells of underground communications. The task of the operating personnel of gas systems and installations is the timely identification and elimination of gas leaks and strict implementation of production instructions for the use of gaseous fuel, as well as the unconditional high-quality implementation of scheduled preventive inspection and repair of gas supply systems and gas equipment.