Properties of water as fuel
The formula of water is known to almost everyone - H2O. It contains two hydrogen atoms (H2) and one oxygen (O2). They are connected to each other by a covalent bond. Here it is worth recalling the essence of any fuel. These are substances capable of oxidation under the influence of an oxidizing agent, which is oxygen.
The function of an oxide in water can be performed by an oxygen molecule (O2). Hydrogen (H2) becomes a kind of fuel. When it burns, it releases 3 times more energy than when using conventional natural gas, and 2 times more than when burning gasoline. It was these properties that formed the basis of the idea of using water instead of fuel.
Components of a hydrogen plant
The design of a heating system operating on hydrogen is quite simple.
The boiler , which plays the role of a heat exchanger, is the main element where hydrogen production occurs.
A boiler operating on hydrogen can be assembled from available elements, and for its operation only ordinary or distilled water is required (+)
The electrolyser is the main operating part of the boiler, where the electrolytic reaction occurs, leading to the decomposition of water into H2 and O2. The element is a reservoir filled with water into which metal electrodes are placed that have maximum current conductivity.
The plates are connected to wires through which electricity is supplied.
A burner is a device that helps heat the coolant in the heating system. Located in the combustion chamber, a spark is supplied to ignite it.
The burner valve is a special part located at the top of the device. Thanks to this part, the H2 that rises to the top easily overcomes the barrier inaccessible to other released substances and enters directly into the burner.

Factory hydrogen boilers are equipped with a control unit. The panel displays voltage and current indicators, a power regulator and levers for setting other operating parameters
Pipeline – communications that extend from the unit and are used to supply heat to all rooms of the house. For piping, heating pipes with a diameter of 25-32 mm are used. When laying, the fundamental rule is observed: the diameter of each subsequent branch should be smaller than that of the previous one.
Is there an everlasting log?
In reality, this is not a log, but an ordinary metal tank (pipe), welded on both sides. On top along the entire length there are holes in it for steam to escape. The pipe itself also has a hole that can be closed using a valve after the entire volume is filled with water.
You can use cold, but hot will heat up faster. How the device works:
- The tank is placed at the very bottom of the stove. On the left, right and top they cover it with ordinary logs. The stove is lit.
- When heated to a high temperature, water vapor begins to escape from the pipe.
- It enters the burning coals, mixing with air. The specific heat capacity of such a mixture is 2 times greater than that of ordinary air. Water vapor has a heat capacity of 2.14 kJ/kg·K, and air - 1 kJ/kg·K.
The results of such an experiment, according to the statements of those who conducted it:
- Black soot comes out of the smoke. This is explained by the reaction of carbon particles with oxygen.
- The flame becomes more intense, with long tongues.
- Wood burns longer: 1 hour 40 minutes. compared to 1 hour 10 minutes. when burning without an eternal log. Time increases by 40%.
Operating principle of the heating unit
Due to its activity, H2 does not occur in nature in its pure form, but it is quite easy to isolate it from ordinary water by electrolysis, which also releases gaseous oxygen.
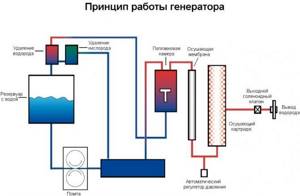
In the presented image you can see a schematic design of heating equipment operating on hydrogen, indicating all structural elements (+)
For the heating device to function, it is first necessary to obtain H2. This happens in a special compartment reserved for such a reaction. Liquid is poured into the container, into which metal plates are immersed.
They are supplied with an electric current of specially selected purity, under the influence of which H2 and O2 are released, as well as water vapor as a by-product.
The resulting mixture is passed through a special device - a chemical separator, with the help of which it is possible to isolate hydrogen, separating it from other impurities. The purified gas is supplied to the burner, on which a valve is installed.
It prevents H2 from moving to the other side, which prevents an explosion. In this case, oxygen and water vapor are released into a special container through another system.
Next, hydrogen gas passes through the protection unit and enters the combustion chamber. Here it reacts with gas in the presence of a catalyst, resulting in the formation of heat, which enters the home heating system through a heat exchanger.
The water vapor released in the chamber is returned through a specially designated channel to the reservoir with the electrolyte, thus using the recycling process.
Power adjustment is carried out using specially equipped channels, the number of which can reach six. Each of these devices contains a catalyst inside, due to which, when turned on, the process of generating heat starts.
The gas flow, heated to a temperature of 40°C, begins to move towards the heat exchanger located in the combustion chamber.
Thanks to separate designs, the channels can operate independently of each other, which allows you to turn on only part of them.

The process of producing hydrogen in modern electrolysis devices is fully automated. The only manual process is pouring water into the system
Modern models are also equipped with various devices, for example, water level indicators and pressure sensors, which allows them to operate automatically and respond urgently in unforeseen situations.
Why is it still not drowned with water?
Intermolecular water bonds arise and break much more easily than intramolecular ones. Therefore, it was decided to use them in heat transfer processes. Chemists have experimentally found that the energy of intermolecular bonds of water ranges from 0.26 to 0.5 eV (electron volts).
The problem is that to obtain fuel from water, it must be broken down into its components. In simple words, it needs to be decomposed into oxygen and hydrogen, then burn the hydrogen and get water again. Cleavage is achieved by passing an electric current through the liquid.
When boiling, water does not break into individual molecules, but only evaporates. Heating from normal combustion does not cause any other reactions in the liquid. Moreover, this process also requires a lot of energy, which could be used usefully. Eg:
- burning 1 kg of dry wood with a moisture content of no more than 20% gives about 3.9 kW;
- if the wood moisture level rises to 50%, then only 2.2 kW is released from 1 kg.
The decomposition of water to produce actual combustion requires significant energy expenditure. Much more of it is needed than will be released when the recovered elements are used again as fuel. An approximate ratio can be given:
- 100% of energy is for splitting;
- 75% of energy comes from burning recovered components.
It is the fact that the reverse reaction of released hydrogen and oxygen releases less energy that is the reason why water is still not used as a fuel for cars and other things. Economically, this method turned out to be unprofitable. It is more feasible to make fuel from garbage. It can be liquid, gaseous and solid.
Is there a "water" car?
In 2008, in Japan, a “water” car was presented by Genepax at an exhibition in Osaka. The fuel could be a glass of tap or river water, or even regular soda.

The device split the liquid into hydrogen and oxygen molecules, which began to burn and give the car energy to drive. Today it is known that the Genepax company went bankrupt and closed within a year.
Top 5 Factory Hydrogen Generators
The first company to manufacture and patent the technology for manufacturing a hydrogen fuel boiler was the Italian company Giacomini . It specializes in devices based on environmentally friendly methods of generating energy: geothermal pumps, solar panels and others.

H2ydroGEM is a catalytic combustion chamber, each horn of which contains a substance that accelerates the combustion reaction of hydrogen. Due to this, the process occurs at a relatively low temperature
Currently, similar models are manufactured by American, Chinese, and European companies, but their range is not very wide compared to boilers operating on other types of fuel.
The best factory models of hydrogen systems
Among the most popular models we note:
- MegaTank100 is a generator that runs on electricity from the network. It has a reliable multi-level protection system against overheating and short circuiting, which guarantees safe and productive operation. The cost of the model depends on its configuration.
- STAR-2000 is an expensive unit (>200,000 rubles) with excellent technical characteristics. Despite the fact that this generator consumes minimal energy, it is capable of heating a room of 251-300 square meters.
- Kingkar is a mains-powered device with excellent performance properties. The cost of the model is quite high - about 100 thousand rubles, but it is offset by economical energy consumption.
- H2-2 – Italian “extra” class equipment at a high price (approximately 250,000 rubles). allowing to heat air in large spaces (from 300 m3 and above) with minimal electricity consumption.
- Free Energy - high-quality devices at an affordable price in the range of 15-35 thousand rubles (the price depends on the power and other characteristics). Equipped with a control unit that automates many processes, a multi-level voltage and pressure regulation sensor.
There are also other models in different price categories.
Adding water to regular fuel
Water can be used as fuel for your car as part of regular diesel fuel. This is another assumption that was put forward by “home” inventors. It turned out that when you add a small amount of diesel fuel to a bottle of water, the resulting mixture burns. Moreover, less soot is released, and the combustion process becomes more violent.
Also, during the burning process of a piece of paper dipped into the resulting mixture, a cracking sound appears, but this only indicates the evaporation of the liquid. In addition, shaking does not dissolve the diesel fuel in the water. There will be no homogeneous mixture here. Over time, diesel fuel, like oil or gasoline, collects on the surface.

A similar experiment was carried out with a tractor, into which diesel fuel and water, mixed in certain proportions, were poured. The unit started up and began to rattle, standing still. But that’s all the energy of such fuel is enough for. And there is a high risk that the engine will fail.