On the eve of winter cold, the question of how to prepare water for heating systems becomes especially important. Proper water preparation is doubly important for owners of private suburban areas that are not connected to a heating plant and receive water from wells or wells. If the water is hard and contains foreign impurities, for example, iron or manganese, this can lead to failure of not only plumbing fixtures and household electrical appliances, but also damage to heat exchangers, corrosion of pipelines and radiators.
Heating system of a country house.
The first and most important stage of work
The main thing that should be done before planning water treatment measures for a heating system is to conduct a chemical analysis of the composition of the water.
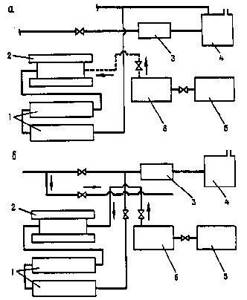
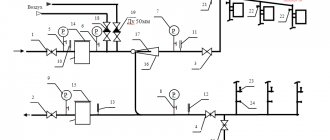
Known (a) and proposed (b) schemes for preparing water for heating: 1 - water heater; 2 — steam-water heater; 3 - refrigerator; 4 - nutrient tank; 5 - high pressure manifold; 6 - low pressure manifold; steam; condensate.
You can carry out tests at home using test kits for aquariums (they are sold at any pet store). However, in order to obtain more accurate values and most effectively prepare water for heating, you should use the services of a certified laboratory.
Water for analysis is collected in a 1.5 liter plastic bottle from non-carbonated drinking water. It is unacceptable to use bottles of sweet carbonated water and other drinks. The cork and bottle are washed well with the same water that is taken for analysis; no detergents should be used. The water is first drained for 10-15 minutes to prevent stagnant water from entering the sample, as this may affect the test results.
To prevent water from being saturated with oxygen dissolved in the air, it is drawn in a thin stream so that it flows down the wall of the bottle. Water is poured under the neck. The bottle is tightly wrapped with a cork to prevent air from entering under it. Oxygen causes chemical processes to occur, and this can also affect test results. If it is not possible to immediately take the samples to the laboratory, then the water can be stored in the refrigerator (not in the freezer!), but no more than two days.
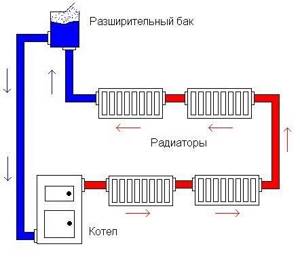
Heating system.
A comprehensive water analysis includes checks for the following indicators:
- rigidity;
- iron;
- manganese;
- pH (degree of acidity);
- permanganate oxidability (indicates the presence of organic substances in water);
- mineralization;
- ammonium;
- oxygen saturation;
- turbidity, color, smell.
If necessary, samples are taken for the presence of microorganisms. Some of them, such as legionella and amoeba, not only can cause serious harm to health, but can also settle inside pipes, forming a slimy microbial film. This promotes corrosion and degrades heating quality.
Related article: Milk wallpaper
What problems exist when using antifreeze fluid in heating systems?
Problem #1
Since water and antifreeze have different physical characteristics, when designing a heating system, it is necessary to take into account whether one or another liquid will be used. Basic calculations are made, of course, for water. If you plan to use antifreeze, you will need to change some system parameters:
- boiler power;
- increase the pressure of the circulation pump by 60%;
- increase the volume of the expansion tank by 50%;
- increase the thermal power of radiators by 50%.
Problem #2
Antifreezes based on ethylene glycol have one feature - they “do not like” system overheating. For example, if at any point in the system the temperature exceeds the critical temperature for a given brand of mixture, ethylene glycol and additives will decompose, resulting in the formation of solid precipitates and acids. When precipitation falls on the heating components of the boiler, carbon deposits appear, which reduces heat transfer, stimulates the appearance of new precipitation, and increases the likelihood of overheating.

The acids formed during the decomposition of ethylene glycol react with the metals of the system, as a result of which the development of corrosion processes is possible. Decomposition of additives can cause a decrease in the protective characteristics of the composition in relation to seals, which can cause leakage at the joints. If the system is zinc coated, the use of antifreeze is not permitted. When overheated, increased foaming occurs, which means that airing of the system is guaranteed. Therefore, in order to eliminate all these phenomena, it is necessary to strictly control the heating process. Since boiler manufacturers do not know the physical properties of the coolants used (except water), they exclude their use.
Water too hard and too soft
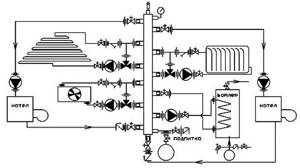
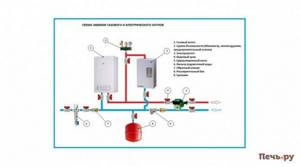
An example of a boiler room diagram for a heating system that provides quick installation and comfortable heating and hot water preparation in a private house, cottage, or country house.
Normal hardness values are 7-10 mEq/l. If this value is exceeded, it means that the water contains an excess amount of calcium and magnesium salts. When heated, the salts form a precipitate known as scale. Accumulating inside pipes and radiators, scale interferes with heat transfer and contributes to wear and tear of the heating system.
The most affordable way to soften water is boiling. During heat treatment, carbon monoxide is removed, and therefore calcium hardness is significantly reduced. However, some calcium remains in the water, so boiling will not completely remove hardness.
Another cleaning method is to use filters with scale inhibitors (neutralizers), such as lime, caustic soda, soda ash. Hard water is also passed through ion exchange resin filters, replacing potassium and magnesium ions with sodium ions.
The use of magnetic softeners is a reagent-free method of water softening. Under the influence of a magnetic field, the properties of water change so that potassium and magnesium salts lose their ability to form as solid sediment and are released in the form of loose sludge. However, salts still remain in the water and need to be removed. In addition, this method is not as effective at water temperatures above 70-75 degrees (that is, the temperature common for boilers, water heaters and boilers).
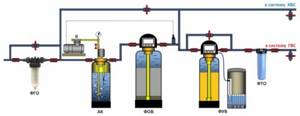
Rough cleaning and deferrization of all water, water softening for heating and hot water supply (DHW) systems.
Reverse osmosis purification involves forcing water through a special membrane that traps harmful substances. This allows you to completely remove calcium and magnesium salts, which cause scale. But this method also has disadvantages: the high cost of treatment equipment and the consumption of a large amount of water during cleaning (for 1 liter of clean water, approximately 2 to 10 liters are drained into the sewer).
Too soft desalted water, for example, rain or melted water, is no less harmful to the heating system than hard water, since the calcium salts contained in the water neutralize acidic reactions, slowing down corrosion. Therefore, before using rain or melt water for the heating system, you should let it settle for several days and refill it only after making sure that its pH level is in the range of 6.5-8, but not lower. This is especially important if the wiring was made from non-galvanized pipes, which were initially susceptible to corrosion.
Related article: Roller blinds for plastic windows without drilling - gentle systems
What else to look for when choosing
All softening filters for water of a certain type have the same principle of operation and differ only in the quality of work, less often - in practically insignificant design features. Therefore, there are no specific selection criteria. However, we recommend paying attention to:
- productivity - also throughput, measured in m 3 / hour or l / hour;
- size - magnetic softeners are slightly larger than the diameter of the pipe, while polyphosphate filters require a lot of space around the pipe;
- thread diameter - must match the diameter of the pipes used in the planned installation location;
- manufacturer - it is better to choose a filter from a well-known manufacturer, preferably in specialized retail outlets that can provide repairs or replacements during the warranty period.
Methods for deferrizing water
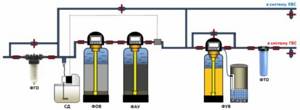
Rough cleaning, reagent disinfection and deferrization of all water, elimination of excess chlorine and sorption additional purification of water, water softening for heating and hot water systems.
The maximum permissible iron content in water for technical needs, in particular for the heating system, should not exceed 1 mg/l. The ideal value is 0.3 mg/l. An excess of iron leads to silting of the internal surfaces of pipes and the proliferation of bacteria in the ferrous sediment, which occurs especially actively already at 30-40 degrees Celsius. This leads to rapid wear and tear of the hot water supply and heating system.
The easiest way to deferrize is settling. Under the influence of oxygen, the iron contained in the water oxidizes on its own, forming a rusty sediment. To carry out iron removal yourself, you will need a large tank with a capacity of 200-300 liters and a device for pumping oxygen: a spray unit or a compressor (for small tanks a regular compressor for aquariums is suitable).
To deferrize water, the same method is quite applicable as to soften it - using the reverse osmosis method. Filters with ion exchange resins are also used. To prevent the proliferation of iron bacteria, chlorination (50 mg/l) is used, but first you should find out how resistant the installed water supply facilities are to chlorine.
If the iron content in water is over 5 mg/l (which is not uncommon for water from wells), then filters with glauconite sand enriched with manganese oxide are used for purification. After passing through a filter medium that serves as an oxidation catalyst, the water gets rid of iron, manganese and hydrogen sulfide, which precipitate. When such a filter becomes clogged, it must be washed with solutions that restore its oxidizing capacity (potassium permanganate solution). It should be remembered that with this cleaning method, harmful chemicals are discharged into the sewer system, so it can only be used if there is a centralized sewer system on the site. Removal of mechanical impurities, manganese, microorganisms, oxygen
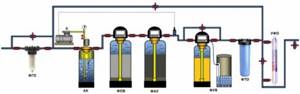
Rough water purification, removal of dissolved gases, deferrization, sorption purification, water softening and disinfection.
To remove foreign impurities (sand, peat fibers, phyto- and zooplankton, fine clay, dirt, organic substances, etc.), various mechanical filters equipped with washable or removable cartridges are used. For very heavy contamination, pressure filters with granular loading are used (quartz sand, expanded clay, activated carbon, anthracite).
The most obvious sign of the presence of manganese in water is a black precipitate. Its concentration rarely exceeds 2 mg/l, but already at a concentration of 0.05 mg/l, manganese can deposit on the walls of pipes, gradually clogging them. Typically, manganese is dissolved in water along with iron, so that with the de-ironization of water, demanganation also occurs simultaneously. To remove manganese, filters with ion exchange resins are used.
Related article: Leveling the floor with a self-leveling mixture: screed and drying time of the self-leveling mixture, preferably gypsum and cement
To disinfect water, that is, remove viruses, bacteria, protozoan microorganisms, ozonation, chlorination, and irradiation with ultraviolet rays with a wavelength of 200-300 nm are used.
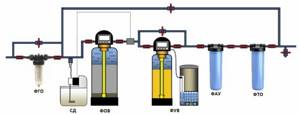
Rough cleaning, reagent disinfection and iron removal, water softening, elimination of excess chlorine and sorption purification of water, final fine cleaning.
The ultraviolet irradiation method is the safest method of water disinfection among the above, since it does not affect its chemical composition, affecting only harmful microorganisms. Water disinfection using UV units occurs in a few seconds.
The corrosive activity of water strongly depends on the presence of oxygen dissolved in it. The dissolved oxygen rate for closed and open heating systems is the same and is no more than 0.05 mg/cub.m. To reduce the oxygen content in water, deaeration units and columns are used.
To prevent oxygen from entering heating systems in other ways (with air), you need to monitor the overall integrity and tightness of the system and not fill it with water too quickly, as this contributes to the formation of air locks. If pipes made of gas-permeable materials, such as polyethylene or polypropylene, are used, they must be protected with an anti-diffusion layer of aluminum.
Prevention or regular cleaning: choosing the optimal operating mode
You should study the scale protection in more detail. Sand scratching the walls of the pipes is noticeable. It is not difficult to separate it with a simple filter for cleaning the heating system. Salts are initially present in a dissolved state. They do not interfere with transparency and, at low concentrations, are indistinguishable in taste.
Even at low concentrations (less than the permissible norm of 7 mEq/liter), such impurities are actively converted into solid particles. The process accelerates in the hottest areas of the system. Scale blocks the movement of liquid, which is accompanied by thermal destruction of the functional unit. The occurrence of such malfunctions during the heating season is accompanied by large financial costs to restore operability. The worst thing is when the accident occurred in the absence of the owners. In this case, ice plugs can rupture the pipes.

It is clear that any attentive person will pay due attention to this problem. However, it is not always possible to make the right decision. Difficulties arise when choosing suitable equipment for cleaning a heating system from a large number of different thematic offers.
To make things easier, some users use cleaning. Such services are offered by specialized service companies. They recommend flushing the gas boiler heat exchanger every 2-3 years, and in individual cases more often. To perform the procedure, they use aggressive acid-based chemicals that can destroy durable calcium formations.
You can perform these same operations yourself. However, we must remember that you will need high-quality specialized equipment, an accurate choice of reagents, and strict adherence to the rules of technology. As a result, the total costs may be higher than if you turn to professionals.
During the reproduction of the technique, experienced specialists monitor the process of removing impurities by changing the color of the liquid. A special reagent is added to the radiator, which acts as an indicator. However, there is no way to check the condition of the pipe walls. Acid destroys metal and solder joints. This negative impact cannot be quickly verified. As with scale, the likelihood of accidents increases.

Does it make sense to create new problems during cleanup? It is much wiser to prevent scale formation. This approach will help maintain the durability and impeccable functional condition of heating equipment - radiators, pipes, boilers.
Antifreeze for heating
In addition to water, special non-freezing liquids - antifreeze - are poured into heating systems. Usually these are aqueous solutions of polyhydric alcohols. Not so long ago, glycerin-based antifreeze appeared on our market. So now there are three types of non-freezing liquids for heating systems.
Types of non-freezing liquids and their properties
Antifreezes are based on two substances: ethylene glycol and propylene glycol. The first is cheaper, freezes at lower temperatures, but is very toxic. You can get poisoned not only by drinking, but even by simply getting your hands wet or inhaling fumes. The second non-freezing coolant for the heating system is based on propylene glycol. It is more expensive, but safe. Sometimes it is even used as a dietary supplement. Its disadvantage (besides the price) is that it loses fluidity at higher temperatures than propylene glycol.

Ethylene glycol coolant is very toxic
Despite their high toxicity, ethylene glycol coolants are more often purchased. This is most likely due to the price - propylene glycol is twice as expensive. But ethylene glycol antifreeze in its pure form is also chemically active, can foam, and has increased fluidity. Foam and activity are combated with additives, and increased fluidity is not corrected in any way. Paired with toxicity, it is a dangerous combination. If there is the slightest possibility somewhere, this antifreeze will leak. And since its vapors are poisonous, this will not lead to anything good. Therefore, if possible, use propylene glycol.
Another important drawback is that ethylene glycol reacts very poorly to overheating, and overheating occurs at a fairly low temperature. Already at +70°C, a large amount of sediment is formed, which settles on the elements of the heating system. Deposits reduce heat transfer, which again leads to overheating. In this regard, such antifreezes are not used in systems with solid fuel boilers.
Propylene glycol, on the contrary, is chemically almost neutral. It reacts less than other coolants with other substances; overheating occurs at higher temperatures and does not lead to the same consequences.

Propylene glycol coolant is safe, but costs more and freezes at higher temperatures
At the end of the last century, antifreeze for heating systems based on glycerin was developed. It is a cross between ethylene and propylene coolants. It is safe for humans, but does not have a very good effect on gaskets, and also reacts poorly to overheating. In terms of price and temperature characteristics, it is approximately in the same range as propylene coolants (see table).
Features of systems with antifreeze as a coolant
When designing a heating system, the coolant must initially be taken into account. This is due to the lower heat capacity of non-freezing liquids, as well as their other properties. If all the equipment was designed for water, and antifreeze is poured into it, the following problems may arise:
- There won't be enough power and the house will be cold. This is due to the lower thermal conductivity of antifreeze. This problem can be solved with little effort - increase the speed of the coolant by installing a more powerful circulation pump. But in an amicable way, an increase in the number of radiator sections is required.
- In closed systems, the volume of the expansion tank may be insufficient. This is due to the fact that when heated, antifreezes expand more than water. The solution is to install another tank. The total volume should be slightly larger than required (the volume can be taken from the table).
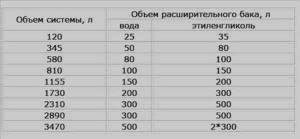
Expansion tank volume for different types of coolant
- If regular rubber gaskets are used, if ethylene glycol or glycerin is used, they will break down and leak after a short time. Therefore, before adding antifreeze, the gaskets in all detachable connections are replaced with paronite or Teflon ones.
As you understand, the best coolant for a heating system is water. It has better characteristics and is several times cheaper. If the heating is in danger of defrosting, you have to fill in antifreeze, but not for automobiles, but special ones for heating. In this case, if you have enough funds, it is better to use propylene glycol. Ethylene antifreezes are a last resort. They are suitable in closed systems, in which special gaskets and automated boilers are installed that will prevent overheating.
To make it easier for customers to navigate, dyes are added to coolants. Ethylene ones are red or pink, propylene ones are green, glycerin ones are blue. After some time, the color may become less intense or disappear completely. This occurs due to thermal destruction of dyes, but does not affect the properties of the antifreeze itself.