Recyclable Products
The use of pyrolysis is widespread. Thus, obtaining petrochemical products is possible only using this method. Coke used in metallurgy is a product of pyrolysis. Landfills for household waste have been developed, where their destruction occurs using thermal decomposition. The good thing about this method is that it is waste-free; this is relevant in the conditions of the Earth’s polluted atmosphere.
Obtaining petrochemical products
When organic complex compounds decompose under the influence of temperature, simple hydrocarbons are obtained. This process produces ethylene and propylene, and various derivatives from them. Based on them, various IUDs are subsequently obtained by polymerization and synthesis. Cracking in petrochemicals occurs at 800–900 degrees.
Wood cracking
The profession of charcoal burners has long been known, who burned wood without access to air underground and obtained charcoal. At a temperature of 500, dry sublimation occurs, which produces valuable products - acetone, resin, acetic acid and methanol. In this case, the carbon remains in a solid state and is called charcoal. Such a product is subsequently used as a high-calorie fuel or an activator of chemical processes.
Pyrolysis begins at a temperature of 200 degrees with the release of carbon oxides. It should also be noted that if the decomposition products are subsequently burned in an air atmosphere, then the total calorie content of their combustion will be much higher than the energy spent on pyrolysis.
Wood chemistry is a science that initially developed only in Russia, and the first cracking experiments belonged to Russian scientists.
Destruction of household waste
The use of pyrolysis to destroy household waste and obtain energy through this is promising. The main obstacle is the content of toxic volatile components in waste - chlorine, phosphorus and sulfur. These are active elements that can bind with other pyrolysis products and create dangerous compounds. Recycling tires and polymer materials allows obtaining secondary products and is economically justified.
During pyrolysis in the apparatus, the processed product goes through the following stages:
- drying process;
- cracking;
- burned the remainder in the atmosphere;
- gas purification in absorbers.
At the same time, the waste incineration plant has different modes and settings designed for a particular process.
To completely process waste, gas products are sent to special absorption plants, where they are cleaned of toxins. The sludge obtained as a result of pyrolysis is a valuable product, as it contains rare elements that are used for further processing.
At the same time, at a waste processing plant you can get:
- thermal energy;
- electrical energy;
- Tire and polymer processing products.
Recycling production during waste sorting will become economical. In the meantime, everything is taken to landfills, even mercury waste ends up.
What is pyrolysis of solid waste, its advantages over simple combustion
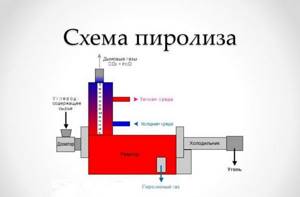
Pyrolysis is the decomposition of heavy organic substances into lighter ones when heated and in the absence of oxygen. In Latin, “pir” means fire, and “lizios” means I decompose, the literal translation of the term is “I decompose with fire.” The meaning of solid waste pyrolysis (see diagram below) is that the compounds that form garbage, when heated, are broken down into substances with a lower molecular weight. As a result of pyrolysis, three main products are formed:
- pyrolysis gas (pyrolysis, pyrolytic gas or synthesis gas) is a mixture of gases capable of burning and non-flammable;
- pyrolysis (pyrolytic) oil and water. Pyrolysis oil has a different composition and can subsequently serve as heating oil or raw material for processing;
- picarbon (solid carbon-containing residue - coal).
During pyrolysis, four processes common to all types of pyrolysis occur: drying of waste (in a drying chamber), dry distillation (pyrolysis), combustion of solid residues, production of pyrolysis gas, pyrolytic oil and carbon residue.
The diagram shows that heating of some stages occurs due to the heat generated during pyrolysis.
Pyrolysis of solid waste has undeniable advantages over waste disposal by incineration. Firstly, there is no environmental pollution, and secondly, the raw material is waste, and it is noteworthy that pyrolysis processes waste that is difficult to dispose of, for example, old tires. Pyrolysis residues do not contain aggressive substances, so they can be stored underground, and such waste is generated in smaller quantities than after combustion. During pyrolysis, heavy metals are not reduced, but go into ash. The resulting products are easy to store and transport. The equipment is not massive, and it is relatively inexpensive.
Application specifics
In an ideal situation, pyrolysis is carried out in a confined space without an influx of air oxygen, with a constant supply of energy. To reduce energy costs for this chemical process, flammable gases generated during pyrolysis are used. As the main equipment used in production, it is necessary to mention gas generators, filters, and cooler units.
Waste in the form of wood chips, sawdust, shavings is placed in a furnace, then the procedure of burning them is carried out with a minimum air supply. Taking into account the dependence between the productivity of the installation and the temperature of the process, the industry uses the option of fast pyrolysis, which involves heating the raw materials to the maximum temperature.
The released gases are cooled, filtered, and pumped into special tanks for subsequent use.
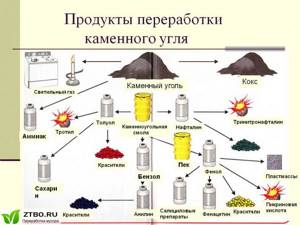
The pyrolysis of coal produces a mixture of valuable products. The peculiarity of this process is the need to heat the mixture to a high temperature. What valuable components for chemical production can be obtained from wood pyrolysis?
First of all, we will highlight coke, which is used in large volumes during the steel smelting process. In addition, the resulting gaseous mixture contains ammonia, which is in demand in fertilizer chemistry.
Aniline is the main component of enamels and paints produced in modern industry. Toluene is a valuable raw material for the production of dyes and explosives.

Features of wood pyrolysis
This pyrolysis is a procedure for burning wood without the presence of air, carried out at a temperature of about 5000 degrees. The valuable products obtained during this interaction are acetic acid, acetone, methanol, and resin. The peculiarity of this chemical reaction is that charcoal can be used as an excellent fuel to accelerate many chemical reactions.
Such pyrolysis is a process that begins to occur at two hundred degrees Celsius, and the reaction is accompanied by the release of a mixture of carbon oxides. With the subsequent combustion of products in an atmosphere of atmospheric oxygen, an increase in the total calorie content is observed.
Wood pyrolysis is a separate section in chemistry that deserves detailed consideration and study.

Types of installations
Pyrolysis plants for processing household and industrial waste have existed for a long time. They convert solid materials into flammable gases. Along with large devices with a productivity of several thousand tons per year, there are small ones that generate electricity.
In the 2000s, models designed to produce biochar appeared.
Waste management complex equipped with a pyrolysis unit, Canada
Technical complexes are assembled from different modules. For example, a device that processes plastics and rubber products may consist of a pyrolysis furnace located above an exhaust gas exhaust system, a chemical synthesis line, a fan, smoke exhausters, and a power unit.
Pyrolysis methods
There are two main methods: dry and oxidative, which are used for recycling different types of raw materials and differ in the method of heating.
Dry method
Pyrolysis occurs without oxygen to prevent combustion or oxidation. If necessary, dehydrating or dehydrating agents are added. Containers with raw materials are heated from the outside. Laboratory installations are equipped with electric heat supply systems.
There are three temperature modes:
- low temperature, or semi-coking (up to 550 °C)
- medium temperature (550-800 °C);
- high temperature, or coking (above 800 °C).
The dry method is suitable for processing and neutralizing hydrocarbon waste. The resulting products are raw materials for the chemical industry.
Oxidative method
The pyrolyzed raw material is heated to 600-900 °C by feeding hot flue gases into a closed container or partially burned. The oxidative pyrolysis method is used for the destruction of solid waste from industrial enterprises and wastewater, processing of plastic, rubber and other materials that cannot be burned or gasified.
Modern methods
- Catalytic low temperature pyrolysis. A new technology for processing fiber composite materials based on resins, which the American company Adherent Technologies is developing to produce carbon fibers. Catalysts are used and the temperature is below 200 °C, so the secondary fibers do not disintegrate and are only slightly inferior in quality to the primary ones.
- Initiated pyrolysis. Designed for processing hydrocarbon raw materials. When certain substances (initiators) are used, the yield of final products increases. For example, the participation of halogen-containing and peroxide compounds in reactions leads to the formation of more ethylene and propylene.
- Thermal contact pyrolysis. The hydrocarbons of the raw material come into direct contact with the catalyst - particles of heated refractory material, molten metal or other coolant. The main advantages of the method are the continuous elimination of unwanted coke accumulations and the ability to supply thermal energy in any quantity.
- Hydropyrolysis pyrolysis. The connections are heated to high temperatures in the presence of water. The pressure reaches 100 bar, temperature - 900 °C. Instead of coke, the share of which is usually about 80%, more gaseous hydrocarbons and about 20% tar are released.
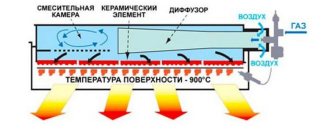
Design of pyrolysis furnaces
Tubular pyrolysis reactors have become widespread in industry. They consist of two parts that differ in the nature of heat exchange - radiation and convection. It is in the radiation section that there are tubular pyrolysis reactors (pyroz coils), heated by the combustion heat of the externally supplied combustible gas in the burners of this section.
In the radiation section, the pyrocoils are heated not directly by the flame of the burners, but by thermal radiation (radiation) from the flame torch (see Planck Formula). and from the thermal radiation of the internal refractory masonry of the radiation section of the installation, directly heated by the flame of the burners.
In the convection part of the installation, heat exchange between the heating gas and combustion products occurs due to convective heat exchange. In this part of the pyrolysis installation, preheating of the raw material, water vapor, and heating to the starting temperature of pyrolysis (600-650 °C) occurs. Gases into the convective part come from the radiation section.
To accurately regulate the temperature in both sections, a smoke exhauster with a regulating damper is installed at the outlet of the unit to control the flow of flue gases.
For energy efficiency, pyrolysis plants are additionally equipped with heat recovery systems - waste heat boilers. In addition to heating the raw material and the water vapor diluting it, the convection part heats the waste water boiler feedwater, and then this water is used to cool the pyrolysis products, while itself heating up. The steam-water mixture obtained as a result of partial evaporation of water is fed into the drum of the recovery boiler. The vapor is separated from the liquid in the drum. The saturated steam from the drum is then additionally superheated in the superheater of the same installation, resulting in superheated medium-pressure steam, which is then used as the working fluid of a steam turbine, which drives a compressor-supercharger for the pyrolysis raw material - pyrolysis gas.
In modern pyrolysis plants, in the convection part there are heating surfaces for superheating saturated steam to a technologically acceptable temperature (550 °C; as the temperature of superheated steam decreases, the thermal efficiency decreases; at high temperatures, the reliability and safety of the installation decreases due to a decrease in the strength of structural steels at high operating temperatures). These measures made it possible to increase the efficiency of heat use in modern models of pyrolysis furnaces to 91-93%.
Pyrolysis of hydrocarbons
Introduction
The process of thermal pyrolysis of hydrocarbon raw materials (oil and its fractions) is the main method for producing low molecular weight unsaturated hydrocarbons - olefins ( alkenes
) - ethylene and propylene.
The existing capacity of pyrolysis plants in the world is 113.0 million tons/year for ethylene or almost 100% of world production and 38.6 million tons/year for propylene or more than 67% of world production (the rest - 30% of propylene production comes from catalytic cracking, about 3% of world propylene production is obtained from by-gases from oil refineries, namely from gases from delayed coking and visbreaking processes). At the same time, the average annual increase in consumption of ethylene and propylene in the world is more than 4% [ when?
][
source not specified 1073 days
].
Along with the production of ethylene and propylene, the process of oil pyrolysis is the main source of production of divinyl, isolated by rectification from the accompanying pyrolysis C4 fraction and distillation of benzene obtained from liquid pyrolysis products.
About 80% of world production of butadiene and 39% of benzene production is carried out by pyrolysis of hydrocarbons [ source not specified 1073 days
].
Pyrolysis conditions and chemical reactions
In industrial conditions, the pyrolysis of hydrocarbons is carried out at temperatures of 800–900 °C and at pressures close to atmospheric (at the entrance to the heated pipeline - pyro-coil
~0.3 MPa, at the outlet there is 0.1 MPa of excess pressure).
The passage time of raw materials through the pyrocoil is 0.1-0.5 seconds.
The theory of pyrolysis has not been sufficiently studied. Most researchers adhere to the theory of a chain free radical mechanism of decomposition during pyrolysis under such conditions.
Conventionally, all reactions during pyrolysis can be divided into primary and secondary. Primary reactions occur with a decrease in the molecular weight of pyrolysis products. These are mainly reactions of the splitting of high molecular weight paraffins and naphthenic hydrocarbons with the formation of hydrocarbons with a lower molecular weight, which is accompanied by an increase in the volume of the gaseous mixture.
Further, secondary reactions of the synthesis of heavier molecules from low molecular weight unsaturated hydrocarbons are possible. These reactions occur mainly in the late stages of pyrolysis.
As the molecular weight of molecules in a mixture of reaction products increases, the volume of gases in the reaction mass decreases.
Basically, the formation reactions of aromatic, condensed aromatic hydrocarbons such as naphthalene and anthracene as a result of the condensation/polycondensation reaction lead to the synthesis of thermally stable aromatic hydrocarbons, including through Diels-Alder reactions.
Also, secondary reactions include the formation of a mixture of various paste-like hydrocarbons with a low specific hydrogen content in the molecules of the compounds, called pitch
.
Pitch, when fired at temperatures above 1000 °C, loses hydrogen as part of the molecules of low-boiling hydrocarbons. The resulting product is usually called pyrolytic coke. But pyrolytic coke differs in many physical properties, in particular in absorption capacity, from coal coke.
The division of reactions into primary (destruction of heavy molecules) and secondary (synthesis of polycondensed aromatic hydrocarbons) is arbitrary, since both types of reactions occur simultaneously.
To reduce the rates of secondary pyrolysis-synthesis reactions, dilute the pyrolysis raw material with water vapor. As a result, the partial vapor pressure of hydrocarbons decreases and, according to Le Chatelier’s principle, a decrease in pressure in the reaction zone will facilitate the occurrence of reactions that occur with a decrease in molecular weight, that is, with an increase in volume, thus ensuring an increase in the yield of decomposition products - the products of primary reactions.
The concentration of water vapor in the pyrolysis process is selected depending on the target product. Thus, for the production of ethylene, butylene, and gasoline, the ratio of steam to raw materials is usually 0.3:1.0, 0.4:1.0, 0.5:1.0, respectively.
Coal pyrolysis products
So, at the very beginning of our article, we mentioned that by pyrolysis from coal you can obtain the following types of products:
- Solid
- Liquid
- Gaseous
Now let's look at each type of pyrolysis product in more detail.
The pyrolysis of coal produces hard coke, which today is used mainly in industries such as ferrous and non-ferrous metallurgy. Coke is a more advanced solid fuel than coal, which is why it is used for metal smelting.
However, coke, although it is the main product of the pyrolysis of coal, is far from the most valuable thing that can be extracted from this natural resource. A by-product of this process is a vapor-gas mixture, which contains many chemical compounds. This mixture is separated by condensation into liquid and gaseous components, from which, in turn, more than 250 chemical compounds can be obtained.
The main liquid product of coal pyrolysis is coal tar, a black liquid product that is a complex mixture of organic compounds. Substances such as:
- Phenols
- Naphthalene
- Anthracene
- Various heterocyclic compounds
- Technical oils
- Synthetic fuel
However, it is worth noting the fact that oils and liquid fuels obtained by pyrolysis of coal are unsuitable for use in internal combustion engines, since they contain many impurities. For this reason, these pyrolysis products require additional purification for further use. And this significantly increases the cost of these pyrolysis products, making their production not very profitable.
The gaseous product of coal pyrolysis is the so-called pyrolysis gas, which is a mixture of flammable gases and various chemical compounds. In many countries around the world, pyrolysis gas is today used as an alternative source of energy, primarily thermal.
While this technology is quite new for us, in some European countries pyrolysis gas has long become a common fuel. In addition, pyrolysis gas, like coal tar, can be used to produce various chemical compounds. Thus, benzene, phenol and other substances are isolated from this gas.
- Comments on the article
Contents of the second block
Modernity
Nowadays, there are two large companies that use the Fischer-Tropsch process in their technology. Most of South Africa's diesel fuel is produced by pyrolysis, followed by oxidation of the resulting products.
This chemical technology gained particular attention after scientists began to look for ways to produce low-sulfur diesel substances that could cause minimal damage to the environment. For example, American companies currently choose coke or coal as feedstock, producing high-quality liquid hydrocarbons

Despite the fact that the pyrolysis process is a mature technology that can be used on a large scale, it is associated with fairly high material costs for the repair and operation of the plant. For many producers, this is a deterrent, as there is a downward trend in world oil prices.
Technological parameters that affect the pyrolysis process
The main technological parameters influencing the results of pyrolysis are:
| № | Helpful information |
| 1 | reactor temperature |
| 2 | residence time of processed raw materials in the reaction zone |
| 3 | concentration of water vapor acting as a diluent |
Due to the fact that the yield of pyrolysis products is significantly influenced by the temperature profile along the length of the reactor, this process is usually characterized by:
- the temperature at the outlet of the pyrocoil, which is designated by the letter t and called the maximum;
- equivalent t-roy te, which is the temperature value of an isothermal reactor, in which the same results are obtained as in a non-isothermal reactor.
In industrial settings, it is defined as the ratio of the average flow volume to the total volume of the reaction zone of the reactor. This also takes into account the change in volume during the reactions.
As the value of m increases, the yield of products such as H2, CH4, coke and benzene increases. Also, the yield values of lower olefins and pyrogas pass through the maximum point. In other words, in order to achieve the highest yield of lower olefins, it is necessary to select the optimal combination of parameter values t, m and f.
In addition, in industrial production a number of other parameters are used that characterize the so-called “rigidity” (process mode). An example of such a parameter is the Linden factor (t·t), which should be 0.06, or the ratio of the amounts of substances such as (H2 + CH4) and C2H4 or C3H6 and C2H4.
Near the inner wall of the pyrocoil, the values of t and m are, as a rule, higher than in the main volume of the reactor. This is due to higher wall temperatures and lower flow velocity along it. In this regard, undesirable secondary reactions occur in the near-wall layer, which cause the formation of coke deposits and a decrease in the yield of the target product.
The higher the concentration of water vapor in the stream, the greater the yield of products such as ethylene, butenes and butadiene, and the lower the yield of aromatic hydrocarbons. However, additional energy costs are required to supply water steam, as a result of which this supply is carried out at certain optimal intervals.
Coal pyrolysis
ContentIntroduction. 3
Theoretical foundations of processes. 4
Pyrolysis. 13
Literature. 14
Introduction
Thermal processing of coal (pyrolysis) refers to processes that occur when coal is heated in the absence of any reagents. Recently, “pyrolysis” has also come to mean processes involving the influence of some additional reagent (hydropyrolysis, oxidative pyrolysis). Thermal processing often refers to the gasification of coal, although additional reagents are also used, most often oxidizing agents, but sometimes hydrogen or methane.
Thermal processing of solid fuels is used to obtain refined carbonaceous solid materials, as well as for liquid and gaseous products. Depending on the purpose of the products, the starting raw material can be almost any coal. As a rule, thermal processing of coal is carried out in the absence of catalysts; There are also no complex recirculation systems, which determines the sufficient simplicity of the hardware design. In this regard, the specific capital costs for thermal processing are significantly lower than in any other coal processing processes.
Coal thermal processing processes were already used at the end of the 18th – beginning of the 19th centuries (production of coal coke, production of refined coals for smokeless combustion, production of illuminating gas, etc.)
A significant part of the thermal processing of fuel processes used today, especially coke production, was formed as a result of the long evolution of technical and equipment solutions and is characterized by relatively favorable costs, energy and environmental indicators.
At the same time, certain restrictions imposed on the processes of thermal processing of coal should be taken into account. All of them are relatively unselective, especially when processing the most common and cheap humus coals. In any process variants, solid, gaseous and liquid products of complex composition are simultaneously obtained, largely predetermined by the elemental composition of the original coal.
Liquid products of thermal processing of solid fossil fuels contain large amounts of organic compounds containing oxygen, nitrogen and sulfur, and therefore cannot be directly used as synthetic liquid hydrocarbon fuel. Therefore, thermal processing of coal cannot be considered as an independent method for preparing artificial liquid fuels. The terms “coal oil” and “shale oil” found in the technical literature are slang in nature and do not reflect the actual state of affairs.
Theoretical foundations of processes
The organic mass of solid fossil fuels is a thermodynamically unstable formation that undergoes profound transformations when heated. The transfer of the laws of transformation of high-molecular compounds to the behavior of coal matter during heating is not sufficiently justified due to the complexity of the structure of this organic mass and the variety of interactions that occur during its processing.
The nature of the thermodynamic transformations of coals is determined by the following features of their structure:
— the presence of a significant number of blocks of 2–10 aromatic and heteroaromatic rings, the π-electrons of which are in a state of conjugation with similar systems;
— the presence of a large number of aliphatic bridges and saturated rings, including sulfide, carbonyl, amine groups, ether and ester bonds;
— the presence of aliphatic side chains, as well as numerous polar groups (carboxyl, hydroxyl, thioxyl, amino groups);
— the presence of heteroatoms – oxygen, nitrogen and sulfur;
— the presence of hydration- and colloid-bound water;
— various donor-acceptor interactions between the organic mass of coal (hereinafter OMC) and mineral impurities.
Naturally, these features, which are inherent in the main mass of all solid combustible minerals, differ significantly for humus and sapropelite materials, for solid combustible minerals of varying degrees of metamorphism. Because of this, the conditions for thermal decomposition and the yield and composition of the products of thermal decomposition of caustobioliths of the peat, lignite and coal stages differ within very wide limits.
Thermal transformations of coal begin at temperatures of about 2000 C. However, already when heated to 1200 C, physically bound moisture and gases adsorbed by coal (carbon dioxide, methane, air components) are released. In this case, no noticeable decomposition of the WMD is observed, although certain changes in its internal structure cannot be ruled out.
At temperatures above 2000 C, a certain amount of water, formed during the thermal decomposition of WMD, as well as carbon dioxide, begins to be released. This is the result of rather complex chemical transformations affecting mainly external polar groups.
In the range of 250-3250 C, the processes of decomposition of coal matter intensify. There is an intensive release of water vapor, carbon dioxide, and a certain amount of hydrogen sulfide and organic sulfur compounds are released. At this stage, the oxygen content in coal noticeably decreases, especially in coal at the early stage of metamorphism. However, even in this temperature range, chemical bonds are split only at the end sections of coal macromolecules. Profound changes in the internal structure of the organic mass of coal have not yet occurred.
At temperatures above 3500C, decomposition of the main organic mass of coal begins. Coal macromolecules are broken down to form short-lived free radicals, which undergo recombination and transform into stable systems. In this case, recombination processes develop in two competing directions: the formation of highly condensed solid products, characterized by a high content of carbon and a low content of hydrogen, and the formation of liquid and gaseous (volatile) products enriched with hydrogen. Between these groups of products, hydrogen is redistributed during thermal decomposition.
Deep decomposition of the organic mass of coal and the release of substances (resins) that are liquid under normal conditions are completed at a temperature of about 5500 C. At 5500 C, a solid residue remains - semi-coke, therefore the thermal processing process ends at a temperature of 500-5500 C, usually called semi-coking. With subsequent heating, processes of further compaction of the charcoal substance occur, as well as the formation and development of microcrystallite graphite-like structures. These processes are accompanied by the elimination of gaseous products - primarily hydrogen, as well as some amounts of ammonia, methane, carbon monoxide, and nitrogen. By approximately 9000 C, the formation of a fairly highly carbonized solid residue - coke - is completed. Heating to higher temperatures - up to 2500-30000 C - leads to the release of coke from heteroatoms, an increase in the orderliness of its structure, and strengthening (especially at temperatures above 18000 C) of graphite microcrystallites.
The nature of the process of thermal decomposition of coals, combined with condensation of decomposition products, is presented in its most general form in Fig. 1.
Fig.1. General scheme of coal pyrolysis: (T – solid phase; L – liquid phase; G – gas phase; 1, 2, 3...n – process stages)
The figure shows the parallel-sequential course of processes with the formation of intermediate unstable products. There is a direct transition to the solid state, a systematic decrease in the mass of the solid residue, a change in the yield of the gas and vapor-gas phases, and the appearance, growth and disappearance of the liquid phase. At all stages of the process, these phases interact with each other and each of them is involved in polycondensation processes, leading to the formation of new liquid, solid and gas phases that undergo transformations in subsequent stages of pyrolysis.
The nature of the destruction of chemical bonds during thermal decomposition largely depends on the rate of heating of the coal. When heated slowly, the weakest bonds are selectively destroyed. At a high heating rate, destruction also accelerates, but lags behind the rate of temperature increase and therefore shifts to the region of higher temperatures. When coal overheats, both weaker and stronger bonds break simultaneously. Therefore, the destruction of the original organic mass becomes more random. In this case, naturally, larger fragments of molecules are formed, from which heavy fractions of the liquid phase of resins are formed, mainly asphaltenes, enriched with oxygen- and nitrogen-containing components.
The largest number of unsaturated and unstable products of coal decomposition are formed in the range of 350-5000 C. In this case, for combustible minerals of low metamorphism, the organic mass of which contains the largest number of less strong chemical bonds, the maximum intensity of formation of unstable products is shifted to the low temperature zone. As the degree of carbonization increases, this maximum shifts to higher temperatures. This pattern is illustrated by the graph of changes in the unsaturation index (iodine number) of coals in the process of thermal destruction presented in Fig. 2.
Fig.2. Changes in the unsaturation of coals during thermal destruction
One of the most significant features of the thermal decomposition of coals is the redistribution of hydrogen between the products of this decomposition. This significantly distinguishes the destruction of coal matter from the thermal decomposition of aliphatic hydrocarbons and most polymers, which during pyrolysis predominantly pass into the gas phase. During the thermal decomposition of coal matter, condensation of cycles occurs with the formation of products enriched in carbon. Thus, the condensed carbonized product is formed by the interaction of free macroradicals and unsaturated molecules obtained mainly from the dehydrogenated or hydrogen-depleted part of the residual mass of coal.
The thermal decomposition of a group of hard coals, which are characterized by a volatile yield of 15-40% and a carbon content of 80-90%, has been studied most thoroughly. A special feature of these coals is the ability to form strong sintered or fused coke during thermal decomposition, and in the temperature zone of 400-4800 C it is in a kind of “plastic state”. It is these coals that serve as the main raw material for the currently most common process of thermal processing of coals - high-temperature coking. These so-called coking coals, due to their physical characteristics, occupy a special position in the genetic series of coals. Among coals that differ in carbon content, they are distinguished by the minimum values of thermal conductivity coefficients, actual density, specific absorption of surfactants from solution and, at the same time, the ability to provide maximum extract yield during high-temperature extraction. In the corresponding genetic series, they are relatively depleted in oxygen and are characterized by a high content of hydrogen bound to carbon.
According to N.S. Gryaznov, the ability of coals of the same degree of metamorphism and similar petrographic composition to transition to a plastic state and sintering is determined by the degree of reduction, i.e., mainly by the ratio of the content of hydrogen and oxygen and their bonds in the structure of the organic mass. This is what influences the coordinated change in a number of specific properties of coals, reaching an extreme for coals with an average degree of metamorphism. The characteristic minimum dielectric constant of coals, for example, is due to a decrease in the number of oxygen-containing functional groups and hydrogen bonds for fatty and coking coals.
It is the fatty coals that are most capable of forming a mobile plastic mass that are characterized by the highest value of the H/O ratio - the hydrogen-oxygen index. And at the same time, the aromatic structures of the organic mass of coals of this type remain relatively slightly condensed. The number of aromatic cycles in the structural units of fatty and coking coals is 3.5-3.7 versus 2.9 for long-flame coals. Therefore, the “liquid” products of the primary decomposition of the organic mass of these coals have significant mobility.
With the help of reduction processes leading to the formation of coking coals, it is possible to explain the increased hydrophobicity of coals, which reaches a maximum precisely in fatty and coking coals. All this leads to the fact that in the temperature region corresponding to the maximum thermal decomposition of the organic mass of coking coals, a significant amount of liquid decomposition products are formed, similar in structure to the original coal and capable of dispersing the solid phase.
The following main stages of the mechanism of transition to the plastic state of coals can be established:
1. redistribution of hydrogen and selective hydrogenation of part of the intermediate products forming the solid phase;
2. formation of a polydisperse system and the emergence of a continuous spectrum of molecular weights of intermediate products;
3. achieving maximum fluidity under conditions of loss of the liquid phase as a result of a decrease in its molecular weight;
4. the origin and development of supramolecular (solid-phase) formations in the plastic mass of coals, its structuring and hardening during aromatization.
Fig.3. Temperature boundaries and plasticity range of typical Kuzbass coals (solid line – intervals of transition to the state of greatest fluidity, number – thickness of the plastic layer)
The temperature range for the existence of the plastic state is different for coals of different degrees of metamorphism (Fig. 3). As can be seen, this range is maximum for fatty coals, and an increase in the degree of metamorphism shifts the region of the plastic state to a zone of higher temperatures.
At every moment, decomposing coal, a changing liquid phase, and a new solid phase coexist in the plastic mass of coal. Thermally processed coal in a plastic state is in a stage of continuous and irreversible changes.
The quality of the resulting coke depends on the properties of the plastic mass and the dynamics of gas release during thermal transformations of coal. The ratio of the rates of decomposition and the formation of new products at all stages of the existence of the plastic state determines the amount of the liquid phase and the fluidity of the plastic mass, and the latter affect the caking ability of coals and the quality of coke.
Hardening of the plastic mass - the emergence and development of a new solid phase - occurs both on existing solid particles as a result of heteropolycondensation of the sorbed liquid phase, and directly in the mass of the liquid phase during the interaction of its components.
With a decrease in the viscosity of the plastic mass, the degree of ordering of the structure and the mutual orientation in space of aromatized macromolecules and their blocks increase. From them the nuclei of a new solid phase are formed. At a sufficiently high concentration of nuclei, bonds arise between them and structuring of the plastic mass occurs. At the same time, its viscosity quickly increases, and a cross-linked solid structure is formed.
With a sufficient amount of non-volatile liquid products, after they have hardened and the plastic mass has hardened as a whole, the coal grains (more precisely, the solid residues of their thermal destruction) turn out to be sintered in the resulting structure (i.e., fused together). The rate of solidification in a series of coals similar in origin and petrographic composition decreases in the sequence G→GZh→Zh→K→OS.
Plastic masses, and therefore sintering processes, are different for different types of coals. Thus, relatively poorly metamorphosed coals containing significant amounts of oxygen (for example, gas coals), during thermal decomposition, form liquid-phase products characterized by low thermal stability and low plasticizing effect. The rate of hardening of the plastic mass is also high. When coking only gas coals, this leads to the production of fine, relatively weak coke.
Plastic mass from fatty and coking coals is more homogeneous in composition and contains fewer low-molecular components. Liquid-phase components are removed from coal grains at higher temperatures and have a good plasticizing effect. The plasticity range is wider, curing proceeds more slowly even with maximum contact between particles due to the high mobility of the plastic mass.
During the thermal decomposition of highly metamorphosed coals, a small amount of liquid products is formed and solidification occurs with a limited contact surface between particles.
Liquid products of the transformation of solid fuels, formed during the primary recombination of free radicals arising during thermal decomposition, are largely similar in structure to the source material and are thermodynamically unstable. Therefore, they undergo a secondary thermal transformation, during which the formation of solid-phase high-molecular material, liquid products consisting of thermally more stable substances, and gas also occurs. Secondary processes and the depth of transformation of primary products naturally increase with increasing temperature and duration of heating (stay in the high temperature zone). Therefore, in almost all currently used technological processes, liquid products are obtained that are formed as a result of fairly deep secondary thermal transformations.
The yield and composition of liquid products largely depends on the H/C and O/C ratio in the original coal. With an increase in the H/C atomic ratio, the proportion of the organic mass of coal that turns into liquid products (tar) increases significantly. In humus coals, the degree of conversion of organic mass into resin and gas does not exceed 20–30%; in sapropelite coals and shale it reaches 70–80%. An increase in the O/C ratio leads to a significant increase in the CO2 content in the gas and the yield of pyrogenetic water, and the appearance of significant amounts of oxygen-containing compounds in the resin (primary shale resin contains up to 50% neutral oxygen-containing substances).
Thus, we can identify the following general trends in changes in the composition of resins and gases during secondary thermal transformations of volatile products:
- reduction in the total resin yield;
- reduction in the amount of neutral oxygen-containing compounds and carboxylic acids, which completely disappear in resins that have undergone pyrolysis at 800 - 850 0 C;
— a significant reduction in the content of phenols (from 25–50% in resins obtained at final temperatures of thermal decomposition of 450–5500 C, to 1–2% at a temperature of 850 0 C);
— a sharp decrease in the content of compounds with a large number of side chains or long (more than one carbon atom) side chains;
— the most stable polycyclic aromatic hydrocarbons and heterocyclic systems accumulate in resins.
During high-temperature (up to 850 0 C) transformation, the composition of the gas changes: the content of methane and higher molecular weight hydrocarbons decreases with a significant increase in the hydrogen content, HCN appears in the gas, formed by the interaction of ammonia with carbon or methane.
Currently, there is no theory that would allow, based on the chemical and petrographic composition of coal and its structure, to predict the course of the pyrolysis process and determine the composition of its products. Therefore, in most cases, the pyrolysis of each specific coal is studied experimentally, and the influence of process parameters on the composition and yield of its products is determined. With the help of theory, at best, it is possible to give a qualitative interpretation of the results obtained and build a more or less adequate model of the process. With such an experimental study, it should be noted, first of all, that the thermal decomposition of coals with varying degrees of metamorphism, and in particular stone or brown coals, proceeds differently. Since during the process of coal metamorphism the loss of the most loosely bound structural groups that make up the macerals occurs, it is clear that coals with a higher degree of metamorphism should be more resistant than young coals. As noted, the thermal decomposition of brown coals begins at a temperature 50–70 K lower than the decomposition of hard coals, and the amount of volatiles released during the pyrolysis of brown coals is significantly greater than during the pyrolysis of hard coals.
In the formation of coke, the decomposition reactions of coal represent only the first stage. After the solid phase has acquired a porous structure due to the release of volatiles, polycondensation and polymerization of part of the released hydrocarbons, which are also part of the coke, occur on the outer surface and on the surface of the pores. If decomposition processes are endothermic (heat of reaction is about 120 kJ/g), then polycondensation processes are exothermic. The processes occurring in the solid phase during coal pyrolysis can be most clearly observed when heating a cylindrical coal sample from one of the ends.
Fig.4. Change in coal density during pyrolysis at a heat flow of 10 W/cm2
Figure 4a shows the change in the density of the solid phase of a bituminous coal sample when it is heated using laser radiation with a power density of about 10 W/cm2. the density of the original coal is 1.33 g/cm3. During the heating process, due to decomposition and release of volatiles, the density of coal decreases to 0.2 g/cm3, and then due to polycondensation it increases again to 0.8 g/cm3 (coke density). Figure 4b conventionally shows the zones that form in a coal sample as the coking process spreads from left to right. In zone I, coal has practically not yet undergone any changes; in zone II, active gas evolution occurs and the density decreases to a minimum value; in zone III, polycondensation occurs, accompanied by an increase in density; in zone IV, the coking process is completed and the density remains unchanged. As the process develops over time, the zones of gas evolution and polycondensation move from left to right, as can be seen in Fig. 4a.
If a small particle of coal, for example, tens or hundreds of micrometers in size, is subjected to pyrolysis, then these zones cannot be formed. Moreover, the course and result of the process will turn out to be significantly dependent on the rate of heating of the particle to a given temperature and on the holding time at this temperature.
Resins formed during the thermal decomposition of coal are, as noted, a mixture of various organic substances, including those with a complex structure. Just like coke, resins are formed as a result of a combination of destruction and polymerization processes. The yield of tar during pyrolysis depends significantly on temperature. The highest yield corresponds to the semi-coking stage (heating to 500 - 550 0 C). With a further increase in temperature, the amount of resin decreases again due to the fact that it undergoes thermal cracking.
Equally important is the duration of exposure at high temperatures formed during the pyrolysis of the vapor-gas mixture. Since the gaseous and vaporous products of coal decomposition are thermally unstable, along with the already mentioned polycondensation and polymerization on the coke surface, a rearrangement of molecules also occurs in the gas phase. Therefore, until the required temperature and depth of coal decomposition is achieved, the vapor-gas mixture should be separated from the solid residue and quickly cooled (“hardened”) to temperatures at which the reaction rate is significantly lower. In this case, it is possible not only to increase the yield of liquid resin, but also to improve its quality.
What has been said about resin largely applies to pyrolysis gases. Primary gases formed during the thermal decomposition of coal, leaving the coke particle, react with it and form secondary gases, which are usually considered as pyrolysis gases. The occurrence of secondary processes is especially active in the case of coking coals, which, as noted, undergo a plastic state during the pyrolysis process. The plastic zone of the plastic state is a two-phase liquid-gas structure through which primary gases bubble, vigorously entering into reactions. The yield and composition of pyrolysis gases, as well as resins, depend on temperature, heating rate and exposure time at high temperature.
Pyrolysis
The yield of liquid and gaseous products during pyrolysis and their composition significantly depends on the heating rate of the coal particle.
However, today there is no consensus on the effect of heating rate on the results of pyrolysis. One fairly plausible explanation is that rapid heating can only be achieved by using very small particles of coal either suspended in a stream of hot gas or mixed with a solid coolant. In the case of a small particle, the volatile products of coal decomposition quickly leave the particle without entering into secondary polymerization reactions with the solid residue or in a reaction in the gas phase with the deposition of condensed products on the surface of the particle.
The effect of heating rate on the mass loss of coal parts was experimentally studied. A small particle of 50 μm in size was subjected to laser heating in a vacuum. By changing the laser power, the particle heating rate was varied from ~103 to 105 K/s. However, no changes in mass loss were detected, indicating that it is the particle size, and not the heating rate, that determines the yield of pyrolysis products. In addition, the yield of volatile products during slow pyrolysis is usually determined in a Fischer retort, where coal particles are placed in a dense layer. In this case, the pyrolysis products inevitably enter into secondary reactions, as a result of which some of the volatile products condense again and the measured yield of volatiles decreases.
When coal softens, volatiles form bubbles inside the coal particle, which move in the quasi-liquid plastic mass of coal to the surface of the particle.
It should also be borne in mind that a high heating rate to a given temperature in itself does not ensure the complete release of volatiles. If, after rapid heating, a coal particle is kept at the reached maximum temperature, the loss of mass (volatile release) continues for a long time.
Literature
1. Chemistry and coal processing / Ed. Dr. H. n. prof. V. G. Lipovich. – M.: Chemistry, 1988. – 336 p.: ill.
2. Gorislavets S. P. Pyrolysis of hydrocarbon raw materials / S. P. Gorislavets, D. N. Tmenov, V. I. Mayorov; Academy of Sciences of the Ukrainian SSR, Institute of Gas. – Kyiv: “Science. Dumka”, 1977. – 307 pp.: ill.
3. Pyrolysis of hydrocarbon raw materials / [T. N. Mukhina, N. L. Barabanov, S. E. Babash and others]. – M.: Chemistry, 1987. – 238 p. : ill.
4. Chemical technology of solid combustible minerals: School for universities / Ed. G. N. Makarov and G. D. Kharlampovich. – M.: Chemistry, 1986. – 496 p.: ill.
5. Lebedev N. N. Chemistry and technology of basic organic and petrochemical synthesis: Textbook for universities, 4th ed., revised. add. – M. Chemistry, 1988. – 592 p.: ill.
Household use
At the household level, pyrolysis technologies are used to produce heat and charcoal, effectively cleaning ovens from difficult-to-remove carbon deposits.
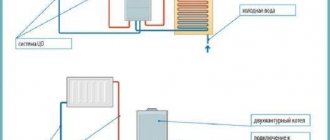
Pyrolysis boilers for heating
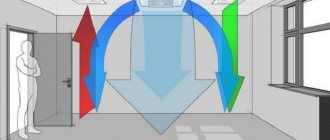
Thanks to their special design, pyrolysis boilers with natural oxygen supply have high efficiency. The raw materials are wood and wood gas. When burned, few substances harmful to the environment are formed. The amount of heat produced depends on the quality of the fuel. Some boilers are designed for wood chips, fuel pellets, coal, and coke.
The main part of the device is two combustion chambers, each of which has its own function. At the top, the raw material is dried and converted into wood gas. Some components of the gas burn there.
Those that are difficult to burn fall into the lower chamber, where they are converted into heat at temperatures above 1000 °C.
Cleaning the oven
Most new oven models are self-cleaning. This happens due to high temperature. Dirt inside the oven carbonizes and falls off on its own or is easily removed. This process, which takes about three hours, is relatively energy-intensive: electricity consumption averages 3-4 kWh. Ashes are removed with a damp sponge after the device has cooled. Before pyrolytic self-cleaning, remove grates, pots, and baking trays.
To obtain charcoal
When processing deciduous or coniferous wood, wood is formed:
- coal,
- vinegar,
- gases,
- resin.
Depending on the temperature, several phases of the process are distinguished. When it rises above 280 °C, a strong exothermic reaction begins and a lot of energy is released. In the last phase (t>500 °C), flammable carbon monoxide and hydrogen are released from the flue gases as they pass through the charred layers. The solid residue is red, black or white coal.
Principle of operation
Unlike traditional solid fuel boilers, pyrolysis boilers use a double combustion cycle. During the thermal decomposition of organic substances, pyrolysis gases are released, the combustion of which leads to a large release of thermal energy.
The use of pyrolysis allows you to obtain more heat from burning fuel. Pyrolysis (gas generator) boilers have two chambers - for burning solid fuel and the released gas.
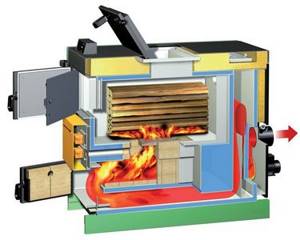
In the first chamber, combustion occurs at a low level of oxygen and high temperature (200-800 ° C), this starts the pyrolysis process. The amount of gases released depends on the raw materials used. The best wood is the one that releases the largest amount of pyrolysis gas when burned.
The optimal thickness of firewood is from 70 mm; in addition to them, you can use pellets or sawdust in an amount of no more than 25%, since they do not provide sufficient combustion power. The operation of a long-burning gas generator boiler occurs according to the following scheme:
- The fuel is placed on the grate (fireproof grate) through the loading window.
- Provide primary air supply to it.
- The fuel is ignited and brought to the mode, achieving the required temperature.
- The supply of primary air is limited by closing the valve, due to which the pyrolysis process begins.
- The pyrolysis gas is supplied by a fan to the secondary chamber, where secondary air is supplied.
- Hot gas burns when in contact with oxygen, releasing a large amount of heat, which heats the coolant in the heat exchanger.
- Combustion products are discharged through the chimney.
Depending on the amount of incoming secondary air, the reaction occurs at different rates. This allows you to regulate the coolant temperature using an automatic valve, limiting the air supply to the afterburner chamber.
With optimal quality of burned wood, the efficiency of long-burning pyrolysis boilers is 85-90%. This indicator decreases sharply with increasing wood humidity, as water vapor reduces the concentration of flammable gases.
How does a pyrolysis boiler work?
The operation of the boiler is based on the principle of pyrolysis, the essence of which is the thermal decomposition of solid fuel at high temperatures under conditions of an artificially created oxygen deficiency. As a result, the fuel smolders, decomposing into a solid residue and pyrolysis gas. The resulting gases also burn, which increases the thermal efficiency of the equipment and makes fuel consumption more rational.
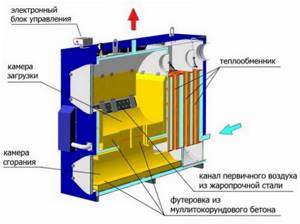
Boiler structure
An additional advantage of the heating boilers under consideration is environmental safety. During the process of pyrolysis combustion of fuel, the released harmful components are mixed with carbon dioxide and utilized. As a result, smoke that does not contain carcinogens and other harmful substances is released into the atmosphere. This feature allows you to heat boilers even with rubber, chipboard scraps and other similar materials.
How does air move in a boiler?
The operation of pyrolysis boilers consists of 4 main stages.
- At the first stage, the fuel is additionally dried and decomposed into solid residue and gases.
- At the second stage, pyrolysis gases are burned.
- In the third stage, the flame is purged and heat is returned to the fuel, which helps release additional heat.
- At the fourth stage, the remaining combustion products are removed through the chimney.
Pyrolysis boiler
Having understood the features of the boiler, we proceed to its manufacture. Let's start by preparing the necessary materials and tools.
Viessman pyrolysis boilerDescription of design
Set for work
- Sheet metal with a thickness of 0.8 mm.
- Fireproof bricks.
- Temperature sensors.
- Grate grate.
- Pipes with a diameter of 32 mm, 57 mm and 160 mm.
- Profiled pipes in the amount of 2 pieces.
- Ash pan door.
- Door for the fuel chamber.
- Fan.
- Flexible burnt wire.
- Bulgarian.
- Grinding wheels.
- Welding machine.
Advantages of pyrolysis-type heating units
- In addition to high efficiency and the ability to control power, the advantages of this equipment include a long combustion duration with one fill.
- Highly environmentally friendly: the combustion process proceeds until the fuel is almost completely decomposed into neutral substances - water and carbon dioxide, chimneys are not overgrown with resins. There are negligible by-products of gaseous harmful substances.
- No smoke in the room. There is no soot formation, the amount of ash generated is minimal. Simple automation makes it easy to operate the unit.
- A variety of fuels: wood, wood waste, coal - this is the advantage of boilers.
- Does not require frequent maintenance: combustion products do not pollute the unit, but periodic cleaning will be required. High-tech automation monitors faults and prevents their occurrence.
Wood pyrolysis
Pyrolysis is the first stage of wood combustion. The familiar flames on burning wood or branches in a fire are formed due to the combustion not of the carbon of the wood itself, but of gases—volatile pyrolysis products. During the pyrolysis of wood (450-500 °C), a lot of different substances are formed, the highest concentrations in the gaseous products of pyrolysis are: methyl alcohol (which is why methanol has the outdated name “wood alcohol”), acetic acid, acetone, benzene, furan, etc. Non-volatile products of incomplete pyrolysis are liquid and paste resins (see Tar). The final product of complete pyrolysis of wood is almost pure carbon (containing some oxides of potassium, sodium, calcium, magnesium and iron as impurities) - charcoal.
This process is used in pyrolysis boilers. The process of wood gasification (pyrolysis) occurs in the upper chamber of the boiler (loading space) under high temperature and with limited air access. The gases formed during this process pass through a high-temperature zone, reach the outlet box and mix there with secondary air.
Pyrolysis of solid waste
Environmentally friendly recycling of waste is one of the key areas of use of pyrolysis. These units make it possible to significantly reduce the negative impact of the anthropogenic factor on the environment.

During the pyrolysis process, bioactive substances decompose and heavy metals are not melted. After thermal decomposition, there is practically no unclaimed waste left in pyrolysis boilers, which makes it possible to significantly reduce the area for their further storage.
For example, by burning 1 ton of tires we pollute the atmosphere with 300 kg of soot. In addition, about 500 kg of toxic substances are released into the air. Processing the same material in pyrolysis plants allows the use of rubber for energy purposes, obtaining recyclable materials for further production and significantly reducing harmful emissions.
It is possible to reduce the harmful impact on the environment thanks to a multi-stage processing system. During the pyrolysis process, waste goes through four stages of disposal:
- initial drying;
- cracking;
- post-burning of processing residues in the atmosphere;
- purification of the resulting gaseous substances in special absorbers.
Pyrolysis plants allow you to process waste:
- wood processing enterprises;
- pharmaceutical industry;
- automotive industry;
- electrical engineering.
The pyrolysis method successfully copes with polymers, sewage waste and household waste. Eliminates the impact of petroleum products on the environment. Excellent for recycling organic waste.
The only disadvantage of pyrolysis units is found when processing raw materials containing chlorine, sulfur, phosphorus and other toxic chemicals. The half-life products of these elements under the influence of temperature can combine with other substances and form toxic alloys.