Pros and cons of this approach
Now, to be honest, you can also pour water into the system, why not! Even from the tap! After all, water is an excellent carrier that can remove excess heat. Nothing will happen to your car instantly, but I think you can drive for about six months, but then problems will start.
- Water is everywhere, in every tap
- It's free
- Combats heat dissipation tolerably
The thing is that ordinary water is not only H2O, it also contains all sorts of salts and other impurities. Also, water does not protect metals from oxidation, rust and subsequent destruction. What else I would like to note is that now the engines of modern cars operate at temperatures above 100 degrees Celsius, for example 105 - 110, and as you know, water boils at 100, so even a working engine will boil and steam. What will this ultimately cause?
Minuses:
- The most harmless thing is scale on the walls of the pipes, a white coating like in any kettle in our apartment.
- The engine, despite the fact that it is made of aluminum alloys, and the cooling system is often made of copper and brass. It will begin to oxidize and rust, especially metal structural elements will be susceptible to this.
- Elements such as the pump, thermostat, metal adapters, etc. will be destroyed, because they are all made of metal.
- Since the water already boils at 100 degrees, air plugs form - there is a high probability that your pipes, radiators, and even the expansion tank itself will rupture; the plug may not save you.

- Do not forget that water freezes already at zero degrees, and at “-3, -4” there will be ice plugs in the system, they will simply rupture the pipes and can even damage the engine block.
Preferences in purchasing antifreeze
There are certain criteria and indicators to which increased attention should be paid:
- The canister of liquid must be solid. As a rule, good antifreeze is not poured into bad containers: the neck is closed with a membrane or seal, and the lid is disposable. Everything should be tightly fitted to each other, there should be no traces of former use;
- Tightness . You can check it by slightly squeezing the canister; if the seal is broken, the canister will hiss and the antifreeze will spill;
- The label is of high quality and is glued well. The numbers, data and barcode have no ghosting and are clearly readable. Basically, technical information is displayed: manufacturer, territorial address and contacts, instructions for use, boiling and freezing points, service life, series number and date of manufacture, composition;
- The translucent canister is very convenient, as it allows you to somewhat inspect the contents. It goes without saying that cloudy liquid should not be purchased. If the canister is shaken and foam forms, it should settle in 3-4 seconds.
When can I fill it?
However, anything can happen on the road, for example, a hose falls off, or the radiator leaks and antifreeze begins to leak out, the level drops! You can’t go on like this, you need to do something. We go to a regular supermarket, or even to the pump - we pour (purchase) water and pour it into our expansion tank without fear. This way, you will be able to make it to the service station, but after that, be sure to drain it, completely flush the system and fill in antifreeze - the one recommended for you.
In this case, this “filling” is justified! You can’t drive with an empty cooling system at all, either a tow truck, or as I indicated. You will prevent the engine from overheating and will make it to the car service normally. Of course, the most ideal thing is to find distilled water, but it is not always on hand.
Distilled water
If you want, it is recommended to dilute the antifreeze concentrate ; it can be poured in a 50/50 ratio. YES and in the short term, it is also desirable for filling (I mean getting to the service station).

The thing is that distilled liquid is just H2O without any impurities (salts, etc.) that can settle in the car’s cooling system. Therefore, it is used to dilute antifreeze and antifreeze.
However, again, it’s clean, you can’t fill it, it will cause all the disadvantages that I listed above, unless there is scale! And so it oxidizes the walls, freezes, and boils beautifully. So you can't!
That’s actually the whole answer, if you compare it with old engines, which were flooded and essentially weren’t afraid, so they were repaired every year, or a maximum of two years. Back then the oils were different, there was no antifreeze, and in general the technology was, to put it mildly, far from ideal.
My late grandfather once told me - if back then those engines had oils and fluids like they do now, we would have built more! So, the people were different, they thought about the country, and not about their pockets as they do now.
Now there is a useful video, watch it
That's all, sincerely your AUTOBLOGGER.
(
17 votes, average: 4.35 out of 5)
Similar news
Engine cooling system pressure. A few words about radiators.
The antifreeze (TOSOL) has darkened and turned brown. Why? Let's analyze the wasps.
How much antifreeze is in the cooling system. Let's count liters
Source
Is it possible to add water instead of antifreeze?
Hello friends! Today we will find out what happens if you add water instead of antifreeze.
As we already know, antifreeze has certain properties and prevents engine overheating. If you fill the cooling system with water rather than antifreeze, the following may happen:
- When working on water for a long time, scale will form in the system, which will gradually clog the cooling system.
- When the engine temperature rises to 100 (and this is the permissible operating temperature) degrees, the water will begin to boil away, an air lock may form, and due to the fact that some of the water will boil away, the engine may overheat, which is fraught with unpleasant consequences.
- If the air temperature drops below zero, the water will freeze, which can lead to damage to the elements of the cooling system: pipes and radiators will burst, the pump and engine block will rupture.
What conclusions follow from this: you can use water instead of antifreeze, but as a temporary alternative to antifreeze (for example, a pipe broke on the road and the antifreeze leaked out, you fixed the leak, but you don’t have antifreeze with you - you can add water, but be sure to change the water upon arrival at your destination for antifreeze with flushing the cooling system) and preferably, of course, distilled, but if this is not available, then any will do, but only in the warm season, when the temperature does not drop below zero; in the heat it is also undesirable to do this - the water may boil away.
CASTLING
What to do if such a leak occurs, and even on the road? First of all, don't panic. Just like in the good old days, clean water will temporarily help out: if you fill it, you will easily reach the centers of civilization with auto chemical stores. Harmful scale does not form very quickly, so you should not be afraid of ruining the radiator. Of course, if it’s winter outside, the water needs to be drained as soon as possible. It is much safer to add any modern antifreeze to the system; It makes sense to rinse it later and fill it with the recommended composition.
Is it possible, on the contrary, to replace water with antifreeze, say, in the old Volga GAZ-21 and drive in comfort all year round? Everything is more complicated here. Firstly, you will have to install an additional expansion tank or drive with an incomplete radiator (we have already discussed the reason above). Secondly, antifreeze, especially when cold, has very good fluidity and the “old lady” seals may not hold it.
Is it possible to fill with water?
In theory, water is no less effective in terms of heat transfer than antifreeze (both ethylene glycol and propylene glycol based). If you pour water into the cooling system, the engine will still operate normally and cool. Maybe the final temperature will be only 2 - 3 degrees higher, but there will not be a significant difference.
But only distilled water is allowed to be filled. In ordinary drinking or technical water there is too much admixture of salts and limestone, which is why scale forms. This sediment practically does not harm the metal, but it can lead to the formation of blockages in the radiator plates, which will deteriorate the cooling efficiency (and over time, microcracks will certainly form).

Accordingly, water instead of antifreeze can be used as a coolant, but for the “health” of the engine this is worse than using antifreeze that is not even of the best quality.
Disadvantages of water for use in the cooling system
Unlike antifreeze, water has the following disadvantages for its use as a coolant:
- provokes metal corrosion (the engine will simply begin to rust from the inside over time);
- does not have washing properties, which accelerates the wear of the circulating pump (the exception is systems with sealed bearings that do not come into contact with the coolant);
- has a lower boiling point (already at 85 degrees “funnels” begin to form, thereby interfering with the normal circulation of the liquid).
One more nuance: active operation of the car with water poured into the cooling system requires regular “topping up” of distilled water, since it simply boils away. And often air pockets can form with it, since in water, unlike antifreeze, a much larger volume of oxygen is “dissolved”. It is this that forms those very “funnels” during boiling. If there is an air lock, the normal circulation of the coolant is also disrupted; the pump simply stops pumping water (which also causes it to overheat).

Also, water has a higher coefficient of volume change when changing temperature conditions. It is because of this that when refueling “up to the neck,” after the engine warms up, steam will definitely begin to ooze from the tank. And if the valve is also faulty, then the expansion tank can be completely damaged.
Coolant service life
During operation, the technical parameters of antifreeze change:
- the number of inhibitors and their concentration decreases;
- a decrease in heat transfer occurs;
- the tendency to form foam increases significantly;
- metals that do not have corrosion protection begin to corrode in the antifreeze environment.
The mileage of the car and the quality of workmanship also largely determine the service life. The change in the properties of antifreeze occurs much faster when air or crankcase gases begin to leak into the crankcase with liquid. In order to avoid this, you need to inspect the pipes and possible leaks more often.
As a rule, antifreeze is changed after a certain number of engine hours, which is prescribed by the manufacturer. But from time to time a situation arises in which the liquid begins to “age” ahead of schedule. The following is observed:
- Closer to the neck of the reservoir, the antifreeze becomes jelly-like, cloudiness and suspension form in the liquid itself, and the electric radiator fan begins to operate much more often. When these “signs” appear, the antifreeze needs to be changed at the first opportunity;
- antifreeze takes on a reddish-brown color. This means the beginning of corrosion. This fluid changes immediately. How to replace antifreeze? Drain the old one, rinse the tank with caustic salt or citric acid solution, and then with water, pour antifreeze according to measurements. It is necessary to carry out work in a respirator, since the ethylene glycol mixture is harmful to the respiratory tract. Replacing antifreeze with antifreeze is carried out only after flushing the entire system with acid. These events are a typical answer to the question: how to change antifreeze and where to fill antifreeze.
Is it possible to mix antifreeze with water?
Water instead of antifreeze is not the best option. Can antifreeze be mixed with water? Experts strongly advise against doing this. This option is allowed only for propylene glycol antifreeze with a crystallization temperature of around -65 degrees.
This composition can be diluted with distilled water in a ratio of 1 to 1 (in this case, the freezing point of the resulting coolant will be around -20 degrees). But it is also recommended to refrain from such mixing (the exception is if such procedures are allowed by the antifreeze manufacturer, as indicated in its instructions).
WHO IS TOSOL
The developers once borrowed the first three letters of the name “Tosol” from a sign above the door of the development department: “Technology of Organic Synthesis” - TOS. And the ending “ol” comes from the chemical terminology used to refer to alcohols (methanol, ethanol, etc.): ethylene glycol is also an alcohol. For some unknown reason, an illiterate opinion has since spread: they say that antifreeze is for “normal” cars, and “antifreeze” is for VAZ cars and others like them... Once again we repeat: “antifreeze” is just one of the types of coolant, but not some special class of chemicals at all! Another thing is that the name has become a household name and is often used when labeling liquids of unpredictable quality. But this has nothing to do with chemistry as a science.
Which antifreeze is better?
The main thing in antifreeze is the correct proportion of additives, which prevent corrosion, changes in the viscosity of the coolant, scale formation, and foaming. Therefore, you should simply use high-quality antifreeze from reputable manufacturers.
Which is better: propylene glycol or ethylene glycol? The difference between them in terms of performance characteristics is minimal. But propylene glycol is not produced in the Russian Federation, so it costs more.

So, is it possible to add water instead of antifreeze in the summer? Yes, the car will work normally. But the consequences of using such a coolant will definitely make themselves felt sooner or later. In the best case, scale will simply appear in the radiator; in the worst case, the corrosion process will be activated, which can only be stopped at an early stage by thoroughly flushing the engine cooling system.
It is not recommended to conduct such experiments; absolutely all car manufacturers indicate that it is necessary to use antifreeze (the brand is selected depending on environmental conditions).
Source
What is the difference between antifreeze and antifreeze?
In order to know the difference between antifreeze and antifreeze, you need to understand their definitions. The difference lies in their terminology.
Antifreeze is a cooling liquid that circulates in an automobile cooling system; the liquid does not freeze at low temperatures. As a rule, it is marked with the freezing temperature: OZh-40, OZh-65.
Tosol is a brand of antifreeze that was developed in 1971 for VAZ cars. Antifreeze replaced Italian-made antifreeze “PARAFLU”. Currently, antifreeze is produced by many companies that produce antifreeze.
This is the main difference: in principle, antifreeze and antifreeze are the same thing, only antifreeze is the common name for all brands. Antifreeze is endowed with its own specific qualities that may not be present in ethylene glycol or coolant-45: color, smell, viscosity, etc. In contrast, one can also note the fact that the difference in heating temperature is different: antifreeze - 97°C, antifreeze - 107°C .
Pour water instead of antifreeze - what will happen?
A frequent question on auto forums: what happens if you add regular water to the system? Today we will look at this issue point by point in simple language.
Is there a danger of pouring water into the cooling circuit?
Let's start from the opposite: why is antifreeze better than water?
?
- Firstly, the boiling point of antifreeze is higher than that of ordinary H2O. Not so much, but those same 15-20 degrees of difference make a difference. After all, the cooling system always works “on the verge of a foul” - right at the boiling point of 95-110 degrees.
- Secondly, coolant contains a lot of specialized additives: anti-foaming, antioxidant, anti-sediment, and many others. Obviously, this entire set is not in the water. This means that with prolonged “savings” on the cooling system, if you ride on water, the metal components of the cooling system will very quickly begin to corrode and become overgrown with deposits.
- And thirdly, if force majeure happened in winter and you replaced the leaked antifreeze with water, the next morning you will most likely find burst pipes, or even the radiator itself. It’s simple: frost hit at night, the water froze and melted everything it could. By the way, this is why on old cars (the well-known 21st Volga, for example), in severe minus conditions, the driver drained water from the cooling system every day in the evening, and refilled it again in the morning.
And if you fill it for a short time.
In case of force majeure, you can also add water. From one-time use of water (especially if it is distilled), CO will not fail and will not even be damaged in any way. But by one-time use I mean just getting to the nearest repair place. Well, prolonged use of such an “emergency” solution will not end well.
And here
I explained in simple words and with illustrations how a car’s cooling system works in principle.
Source
Cooling system and water
Let me remind you a little, why do we need a cooling system? What is its main function?
YES, everything is simple - it removes excess heat from the engine so that it does not overheat and it does not jam. With a liquid system, the engine life has increased significantly.
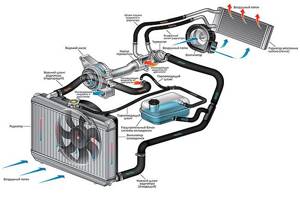
To prevent the coolant from stagnating in one place and boiling there, it needs to be pumped through the system - this is done by a pump (a special pump, belt-driven from the crankshaft). Well, excess heat needs to be dissipated somehow, this is done by the radiators of the car , the main one and the stove (in the cabin, thanks to it we are heated in the winter).

And here’s the thing - the car is completely indifferent to what kind of liquid will remove heat from the engine, the main thing is that the system is as efficient as possible.
I will say more - the heat capacity of antifreeze is about 3.5 kJ/(kg deg), and that of water is about 4.2 kJ/(kg deg) , that is, hypothetically, water is much more suitable for heat removal. But there are also disadvantages (more on them below).